| |
Science of Lattices and Crystals |
| |
|
 |
Before I get started: These two modules give a first idea about lattices and crystals
without all the formal stuff coming up here:
|
| |
|
| |
Bravais Lattices |
 |
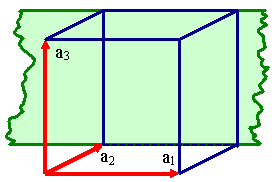
Any crystal lattice can be described by giving a set of three base vectors
a1, a2, a3. A lattice is
formed by generating an infinity of translations vectors
T = ua1 + va2 + wa3
with u, v, w, = integers. The end points of all possible translations vectors define the lattice as
a periodic sequence of points in space.
|
|
 |
If you have some lattice and move it by any translation vector you care to construct, you
have exactly the same lattice once more. In other words: crystal lattices show a translation symmetry! For a long time, the words "crystal"
and translation symmetry were seen as obvious synonyms - until the discovery of quasi-crystals in 1982! |
 |
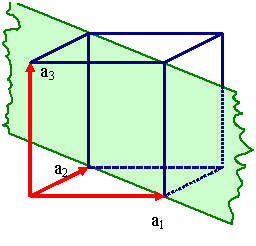
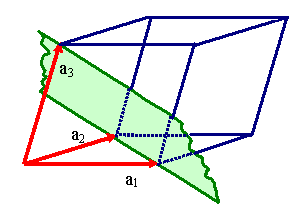
Unfortunately, one and the same lattice can be defined by many different sets
of vector triples as illustrated right below. |
| |
| |
| |
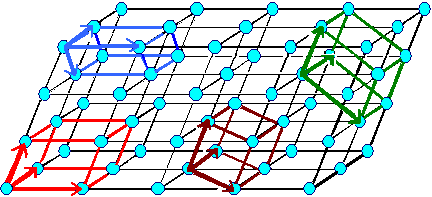 |
Definging a lattice by a vector triple a1, a2,
a3.
There are, however, many different vector triples
that define the same lattice. |
|
| | |
|
|
 |
It is far easier to use some special lattices
instead of just one general type. The thing to do is to go for symmetries as
the distinguishing criterion. That's what Bravais did, showing
that with 14 Bravais lattices all possible cases can be represented. All material
scientists know that "magic" number 14 but very few know how it is derived. I don't know details either
but I know it is an exercise in set theory.
Note that a lattice is a
mathematical construct, a succession of (infinitely small) mathematical points in space.
A perfect drawing of such a lattice thus would show nothing at all. Instead of points,
I use little blue spheres here. They are connected with lines but only to "guide
the eye".
These blue spheres are not representing atoms when a lattice
is shown. More about figures to lattices and crystals in this link. |
|
 |
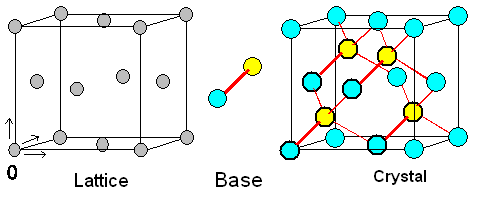
If one wants to make a crystal,
one assigns a so-called base of
atoms to a lattice point. If that base happens to consist of just one
atom or element, we make an element crystal. A schematic
figure of such a crystal with one atom per lattice point then looks exactly like the schematic
representation of a lattice, causing no end of confusion. It is nevertheless something completely
different; see below.
Note that mother nature has not made a cubic primitive element crystal.
|
| | |
|
| |
Bravais Lattices and Their Parameters |
| |
|
|
Cubic
a1= a2 = a3 = a = lattice constant
a = b = g =
900 |
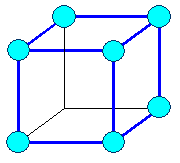
cubic primitive |
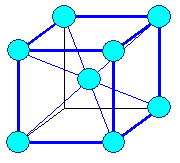
cubic body centered
(bcc) |
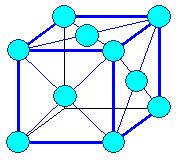
cubic face centered
(fcc) |
Tetragonal
a1= a2
¹ a3 a = b
= g = 900 |
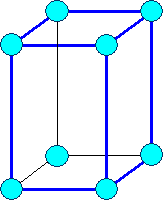
tetragonal primitive |
tetragonal body
centered | |
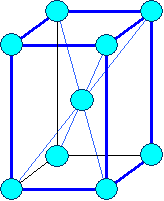
Hexagonal
a1= a2
¹ a3 a = b
= 900,
g = 1200 Typical: a3 = c |
Hexagonal (hex)
| (expanded to show hex symmetry) | |
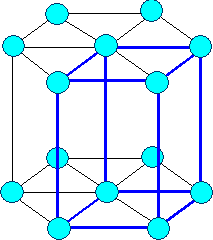
Rhombohedral or Trigonal
a1= a2 = a3 a = b
= g ¹ 900 |
rhombohedral |
| |
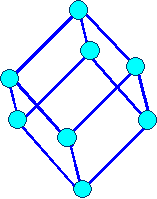
Orthorhombic
a1 ¹ a 2
¹ a3 a = b
= g = 900 |
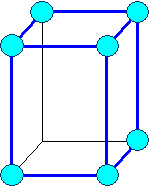
orthorhombic primitiv |
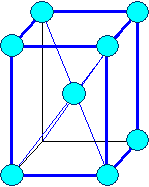
orthorhombic body centered |
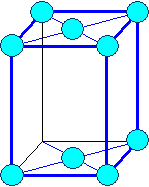
orthorhombic base face centered
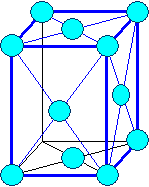
orthorhombic face centered |
Monoclinic
a1 ¹ a2
¹ a3 a = b
= 900, g
¹ 900 |
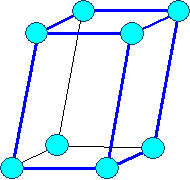
monoclinic primitive |
|
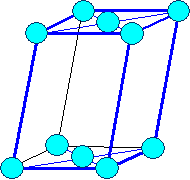
monoclinic
base face centered |
Triclinic
a1 ¹ a2
¹ a3 a
¹ b ¹
g ¹ 900 |
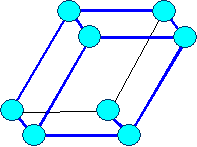
triclinic |
| |
Bravais lattices
(on occasion only "visible" lattice points (= blue circles) are shown) |
|
| |
|
Describing Directions
and Planes by Miller Indices |
 |
Working with lattices and crystals produces rather quickly the need to describe
certain directions and planes in a simple and unambigous way. Stating that an elemental face-centered cubic crystal can
be made by assigning one atom to any lattice point found on "that plane that runs somehow diagonally through the unit
cell" just won't do it.
So William Hallowes Miller invented
a system with a lot of power for doing that in 1839. What we do is to describe any direction or any plane by three
integer numbers, called Miller indices. |
|
 |
How to derive the Miller indices of a certain direction or plane is easy. Here
is the recipe for directions (in 2 dimensions for simplicity); the figure below illustrates it:
- Start the desired direction from the origin.
- Express the direction as a vector given in integer multiples u, v, w of the base vectors.
- Make sure the three integers have the smallest possible value.
- Write the direction as [u v w] or <u v w> (we won't concern
us here with the subtleties involved in using two kinds of brackets).
- Negative integer values are written with a dash on top of the number instead of the conventional "-"
sign. (not possible in simple HTML)
|
| |
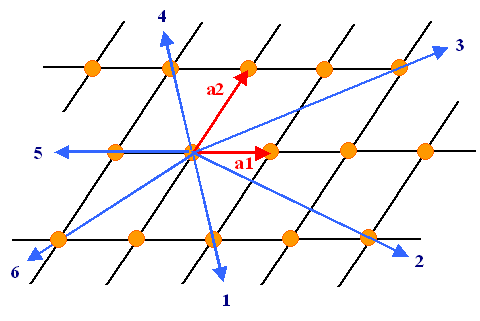 |
| Six directions in a (2-dim) lattice and their Miller indices |
|
| | |
|
| |
 |
Getting Miller indices for planes is a bit more involved. Here is how it's done;
the figure below gives examples:
- Put the origin not on the plane but on a neighboring plane.
- Find the intersection points h', k', and l' of the plane with the (extended) base vectors. If there is none, the value
is ¥.
- Form the reciprocal values of h', k', and l' and call them h, k, and l. If, for example, h' = ¥,
you have h = (1/h') = 0.
- The Miller indices of the plane to be indexed then are {hkl} or (hkl).
|
| | |
 |
Cubic lattice
Intersections at
1, 1, ¥
Indices (110) |
 |
Cubic lattice
Intersections at ¥, 1, ¥
Indices (010) |
 |
Triclinic lattice
Intersections at
1, 1, 1
Indizes (111) |
| Miller Indices for Planes |
|
| | |
|
|
 |
If you wonder why this slightly awkward procedure was adopted, the answer is
easy: You can use the Miller indices directly in a lot of equations needed for calculating properties of crystals. |
| | |
|
| |
From Lattice to Crystal |
 |
Any crystal can be made following this easy recipe:
- Pick a Bravais lattice
- Pick a base, a collection of atoms in a fixed spatial relation (similar and often but not always identical to a molecule
of the substance.
- Put the base in the same way on any lattice point.
|
| | |
|
|
|
 |
| How to make a diamond-type crystal |
| The yellow atom of the base is only shown if it is found inside the unit cell |
|
| |
|
|
 |
The example above shows how to make a crystal of the diamond type. The base consists
of two atoms. In the coordinate system of the lattice unit cell (indicated by arrows), the two atoms have the coordinates
(0,0,0) and (¼,¼,¼).
If the two atoms are of the same kind, e.g. silicon,
(Si), germanium (Ge), or carbon (C), you get a silicon, germanium or diamond crystal.
If the atoms are different,
e.g. from group III or group
V of the periodic table, you get most of the compound semiconductors
like gallium arsenide (GaAs), or indium phosphide (InP). |
|
 |
This looks simple. It is not. It's the point where things get difficult and confusing.
Ask yourself for any still simple crystal: how many atoms are there to a lattice plane? How many atoms are in a base?
Below are three crystals, all have an fcc lattice. Different colors of the circles my
or may not denote different atoms. Can you figure out the bases? If you can, you're ahead of my average third-term student. |
| |
|
|
|
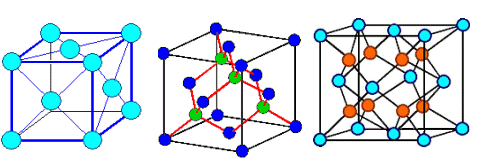 |
| Gold, Aluminum, .. |
Silicon, carbon (diamond), Gallium arsenide (GaAs), InP, .. |
Zirconium oxide (ZrO2), .. |
| Three different kinds of crystals with the same lattice |
|
| |
|
 |
One last thought: Crystals in a general sense, meaning an arbitrary base arranged
in a periodic way, can be found everywhere; here is an example: |
| | |
| |
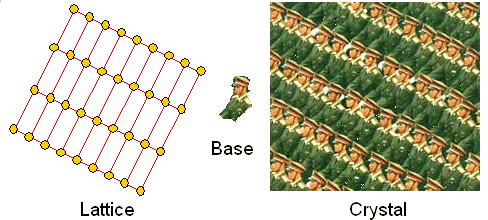 |
| Example of general crystal. |
|
| | |
|
 With frame
With frame

 Periodic Table of the Elements
Periodic Table of the Elements
 4.2.2 Being Iron
4.2.2 Being Iron
 History of Carbon
History of Carbon
 11.2.2 Metallurgy of Celtic Swords
11.2.2 Metallurgy of Celtic Swords
 Group 1 / IA; Alkali Group
Group 1 / IA; Alkali Group
 Group 2 / IIA; Alkaline Earth Metals Group
Group 2 / IIA; Alkaline Earth Metals Group
 Group 12 / IIB; Scandium Group
Group 12 / IIB; Scandium Group
 Group 12 / IIB; Titanium Group
Group 12 / IIB; Titanium Group
 Group 5 / VB; Vandium Group
Group 5 / VB; Vandium Group
 Group VIB; Chromium Group
Group VIB; Chromium Group
 Group 7 / VIIB; Manganese Group
Group 7 / VIIB; Manganese Group
 Group 8 - 10 / VIIIB; Iron - Platinum Group
Group 8 - 10 / VIIIB; Iron - Platinum Group
 Group 11 / IB; Copper Group
Group 11 / IB; Copper Group
 Group 12 / IIB; Zinc Group
Group 12 / IIB; Zinc Group
 Group 13 / IIIA;
Group 13 / IIIA;
 Group 14 / IVA; Carbon Group
Group 14 / IVA; Carbon Group
 Group 15 / VA; Nitrogen Group
Group 15 / VA; Nitrogen Group
 Group 16 / VIA; Chalkogenides or Oxygen Group
Group 16 / VIA; Chalkogenides or Oxygen Group
 Group 18 / VII; Noble Gases
Group 18 / VII; Noble Gases
 Group 1/ I; Hydrogen
Group 1/ I; Hydrogen
 Group 3 / IIIB; Lanthanides or "Rare Earths"
Group 3 / IIIB; Lanthanides or "Rare Earths"
 Group 17 / VIIA; Halogens
Group 17 / VIIA; Halogens
 Magnetism
Magnetism
 Alloying Elements in Detail
Alloying Elements in Detail
 8.4.1 Martensite
8.4.1 Martensite
 Inhomogeneous Deformation
Inhomogeneous Deformation
 Dislocation Science - 1. The Basics
Dislocation Science - 1. The Basics
 Lattice and Crystal
Lattice and Crystal
 Crystal Models
Crystal Models
 X-Ray Diffraction
X-Ray Diffraction
 Phenomenological Modelling of Diffusion
Phenomenological Modelling of Diffusion
 Gemstones
Gemstones
 Beer and Conquering The World
Beer and Conquering The World
 Quasi Crystals
Quasi Crystals
 Science of Deformation
Science of Deformation
 Dislocation Science - 2. The Reality
Dislocation Science - 2. The Reality
 Pictures of Grain Boundaries
Pictures of Grain Boundaries
 Deformation Types
Deformation Types
 Phase Boundary - Advanced
Phase Boundary - Advanced
© H. Föll (Iron, Steel and Swords script)





















