| |
4.2.2 Being Iron |
 |
Again: About 30 % of all the metals, including iron, do not
want to be close packed. They feel that having 12 close neighbors like the vulgar fcc and hcp metals is a bit too much;
8 should be good enough.
Why ? You know as soon as you know the true
name of those atoms. This is secret, of course, so all I can say is that those atoms attract other atoms in all directions
too, but somewhat more in 8 special directions
How can we take that into account when we start stacking balls once
more? |
|
 |
It's easy: Just start with packing your oranges squarely
on the bottom layer instead of hexagonally. What you get then is what we call a "body
centered cubic" crystal lattice, or bcc for short, as shown below. Why this type of crystal lattice is called
"body centered" is obvious if you look at the right-hand figure. You also
see that the special bonding directions are not in the plane where we do square packing but in the diagonals. |
| | |
 |
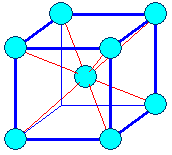 |
| The body centered cubic (bcc) crystal |
| How we stack the atoms when we make an iron crystal. |
The resulting body-centered cubic (bcc) crystal lattice. The red lines show the
preferred bonding directions. |
|
 |
We now covered attractions in all
directions, giving close-packed element crystals and attractions in all directions but with some preferences in special
directions, giving (among others) the bcc structure from above. If, just for the hell of it, we look at the other extreme
of attraction only in some special directions, we get, for example, the diamond structure, the favored structure of most semiconductors. Once more, there is no
choice but to form a crystal when those atoms get together. |
 |
Now we have three basic crystal lattice types,
and if you find that confusing, consider that there are actually fourteen fundamentally
different lattice types or Bravais lattices.
You better believe me on this; I in turn believe Auguste Bravais
who proved that in 1849. Figuring it out by yourself is quite tedious and involves the hated "new math". |
|
 |
So far I have avoided to explain the subtle but essential difference between
the terms "lattice" and "crystal"
but now we need to give this topic a quick look. The science link gives in-depth information, this
link will just illustrate and enlarge on what is following here.
A lattice is a mathematical concept, a repetitive
sequence of points in space. A crystal results when you put atoms on those lattice points. You always can put a single atom
at the lattice points of one of those 14 lattice types, than you get an element crystal. But nobody keeps you from putting
more complex objects like molecules there, however. Take water (H2O) molecules and you make an ice crystal, for
example.
| |
|
| |
Considering that you could put your water molecule on some lattice
point with the oxygen up or with the hydrogen up, and that there might be other ways as well, you now realize that there
are altogether exactly 230 possibilities to make different basic arrangements of entities
like molecules on the lattice points of the 14 basic lattices.
Haha - just joking. I'm rather sure that you could not
figure out that number. I certainly couldn't; at least not off-hand. I can, however, look it up and check if the good people
who are into this, got it right. They did. Just believe me. Use this link
to de-confuse you a bit if you think that's indicated.
If you look at little deeper, and I don't recommend it, even
more possibilities emerge that you definitely don't want to know about. It just serves to show how tremendous complexity—a
DNA crystal, a sword blade, a car body—can emerge from a few very simple principle. |
|
 |
Mineralogists know all about
Bravais lattices and so on, and they like to talk about it. So you should avoid them
at parties if you're not into symmetries
, point groups, space groups , sets,
intersection of sets, and more stuff from the "New Math" that you hated so
much way back in high school. |
 |
And no, it's not almost all the same. If you
look at the table below, where some metals and their lattice types are listed, and compare this to the older list of metals where I differentiated between always ductile behavior, always
rather brittle behavior, or in-between behavior with a brittle-to-ductile transition, you realize that the more ductile
metals are fcc, the more brittle ones are hcp, and the bcc metals are in between. |
|
 |
Do you feel that we are getting closer to some answers? Well, we do, but we also come up with
new big questions: |
|
|
Why do metals crystallize in
different lattice types?
|
|
 |
Why does this happen even for metals that
are quite similar in all other aspects like nickel (Ni; fcc) and cobalt (Co; hcp)? If you read on for quite a while, you are eventually going to find out. |
 |
Right now let's make a little list of the lattice types that
the more prominent metals and elements like to crystallize into. However, first I must
give you a few hints about how to "read" figures like the ones coming up: |
|
 |
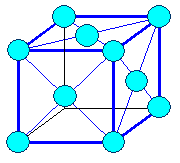
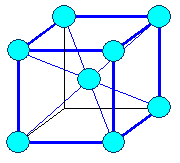
The figures show lattices or crystals.
The little blue spheres in the figures may represent lattice points in space or atoms.
Since true mathematical points are infinitely small and atoms extremely small, the real things are simply not visible and
we need to represent them by some symbol - a circle for example. It is then up to you how you interpret the symbol.
The lines are only there to guide the eye so it can see the spatial relation of the
points that form the lattice. Sorry, we can't do better. If I would draw real (tiny)
points for the lattice and no lines, you just wouldn't see much; check here
.
A crystal results if you put atoms on lattice points! If you symbolize atoms by circles, the resulting crystal
figures looks just like the lattice figures but is showing something entirely different!
More about how to "read" figures like that in this link |
| |
 |
 |
 |
Lattice: fcc
Crystal: fcc, elementary, close-packed
12 next neighbors | Lattice: bcc
Crystal: bcc, elementary
8 next neighbors |
Lattice: hex
Crystal: hcp, elementary, close packed.
12 next neighbors |
Ag (silver)
Au (gold)
Al (aluminum)
Cu (copper)
Si (silicon)
Ge (germanium)
Co (cobalt)
Fe (iron)
Mn (manganese)
Sr (strontium)
C (carbon)
|
Fe (iron)
Cr (chromium)
W (tungsten)
Ta (tantalum)
Mn (manganese)
Ti (titanium)
Zr (zirconium)
Sr (strontium) |
Cd (cadmium)
Co (cobalt)
Mg (magnesium)
Ti (titanium)
Zn (Zinc)
Zr (zirconium)
C (carbon) |
Lattice types / crystal structure of common metals
and some other common elements |
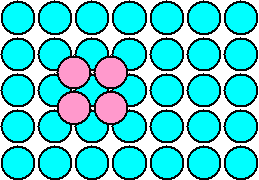
| Blue circles = atoms and
lattice points. Pink circles = atoms only (two atome per lattice point). Note that a hexagonal
close-packed (hcp) crystal results only when you put the "pink" atoms in positions
where there are no lattice points. This is deceiving. You are actually putting an ensemble of 2 atoms onto 1 lattice point.
Details can be found here. |
|
 |
Surprise! Some metals (and many elements not
listed) come in more than one crystal type— in the table they are identified by col or. The
big word for that is "allotropy
" (of course from the old Greek, meaning changing, different kinds, growing into something else). Those crystals
then are "allotropic".
How come? |
|
 |
Before I answer this question, I first need to point out that it is primarily
the temperature that determines what crystal type you will find. The pressure
is also important. Carbon provides for a good example of this: a diamond forms from graphite at high pressure (and temperature). |
|
 |
Pure iron switches from a bcc
structure to an fcc structure at 912 oC (1674 oF) and back again
to bcc at 1394 oC (2541 oF), while Cu or Au stick to their fcc structure at all
temperatures.
Cobalt (Co), manganese (Mn), titanium (Ti), and so forth also undergo some kind of phase change (also called phase
transition) from one lattice kind to another one, if you change the temperature. |
|
 |
Of course, you now need to vent a big question: "How do you know all that!
After all, you can't possibly look at the atomic structure of some metal at very high temperatures with an electron microscope. Not to mention that all of this was known long before electron microscopes
even existed".
True. You are absolutely right. Even today we do not investigate the basic crystalline structure
of materials with an electron microscope. We use interference effects of X- rays.
If you have seen a CD or DVD producing
a spectrum of colors whenever you shine some bright light on, you have seen so-called interference effects. |
|
|
| |
The regular array of tiny grooves on the disc interferes with the light to produce
rainbow colors. Come to think of it, water droplets interfere with light to cause a rainbow. There - you have seen interference
effects!
The regular array of atoms in a crystal does exactly the same thing with X-rays that the regular array of tiny
grooves on a DVD does with light. Shine an X-ray beam onto a crystal and it will produce phenomena akin to what produces
all kinds of "rainbows" with visible light. Recording that with proper detectors and throwing a lot of math at
the data allows to compute the structure of a crystal in minute details.
This is
called X-ray diffraction. X-ray diffraction machines are a kind of mathematical microscope.
They do not produce some direct picture of the crystal, just abstract data - numbers
- about the crystal. The science module will give details. |
 |
But back to the phase changes that we just learned about. The phase
change
of carbon from diamond to graphite,
come to think of it, is simply a change from carbon atoms arranged in a fcc (diamond)
lattice to carbon atoms arranged in an hex (graphite) lattice. The illustration link
shows details. |
 |
It was not before 1772 that Antoine Lavoisier
showed that the only product of the combustion (= burning)
of diamonds was carbon dioxide (CO2), proving that diamonds consist of carbon and nothing else. The story of
figuring that out is interesting enough to rate it's own module. |
|
 |
Of course, nobody believed this kind of nonsense right away. Since Lavoisier
committed other grave errors of judgement, like intervening on behalf of a number of foreign-born scientists including the
(now extremely famous) mathematician Joseph Louis Lagrange,
he was tried, convicted, and guillotined on 8 May 1794 in Paris at the age of 50.
Appeals to spare his life, so that
he could continue his important experiments, was cut short by the judge: "La République
n'a pas besoin de savants ni de chimistes; le cours de la justice ne peut être suspendu." ("The Republic
needs neither scientists nor chemists; the course of justice cannot be delayed") is the infamous quote that goes with
it. There are reasons why scientists are not all that fond of lawyers. |
|
 |
To be sure, in our enlightened age something like that can't happen, our modern lawyers will
see to that. All those scientists (and artists, almost same thing) who in many countries are spending time in jail right
now are there for good reasons, we must assume. |
 |
Change the lattice type and you change diamond to graphite. We have exactly the
same thing for fcc and bcc iron! The bcc room temperature phase (named ferrite,
by the way) is quite different from the high temperature fcc phase (named austenite),
as far as properties are concerned. You are not aware of that because fcc iron only exists at high temperatures and then
everything looks the same (glowing yellow-red) anyway. But properties are very different.
Ask a smith. |
|
 |
But you don't have to ask, you know that. You have witnessed the dire consequences of that
phase transition taking place. When the steel in the World Trade Centers became
so hot that the transition from tough bcc-phase steel to weak fcc-phase steel took place, disaster was unavoidable. |
 |
On the other hand, the bcc-fcc phase transition is one
of the several reasons why iron is such a wonderful material. When the sword smith heats up his iron or steel, he makes
good use of the phase transition bcc-fcc in iron, as we will see |
|
 |
We have come to a point where I can't weasel around the really big
"why " question anymore. |
| |
Why do things happen the way they happen?
|
|
|
 |
What is the guiding principle? I only consider "things to happen" in the inanimate
world, of course, since you already know some of the guiding principles in the animated world. For example, when your wife
asks if she should wear costume A or B to some function, the guiding principle for the ensuing discussion is: You can't
win! Never. Ever. |
| |
| |
© H. Föll (Iron, Steel and Swords script)




