| |
Diamond, and other Carbon Specialities |
| |
Old Stuff |
 |
Here are some figures showing the various phases or modifications of carbon in
elementary form |
|
 |
The simplest crystal is diamond. Here is the crystal structure:
|
| | |
|
|
|
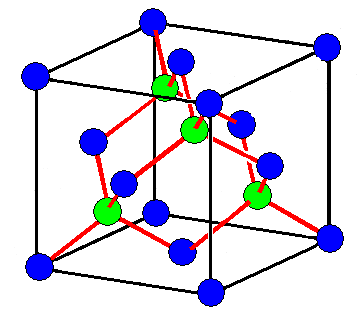 |
| Diamond crystal structure. |
|
| | |
|
|
 |
The blue circles symbolize two
things
- The lattice points of a face-centered cubic
(fcc) lattice.
- Carbon atoms sitting on the lattice points.
The green circles symbolize carbon atoms too - but not lattice points
The red lines
symbolize the very strong bonds between carbon atoms. All atoms are strongly bonded to 4 neighbors and that's why it is
difficult to rip them apart. In other words: Diamond is very hard!
The black lines are only there to guide the eye in
seeing the cubic lattice. They have no meaning. |
|
 |
A diamond emerges if one puts a dumbbell formed by two
carbon atoms on any lattice point of the fcc lattice; just look at the picture to see this. |
 |
The word "diamond" goes back to the ancient Greek "adámas=unbreakable.
It may surprise you that diamond has not only the highest hardness of all (natural) materials, but also the highest
thermal conductivity of any bulk material. That's why a diamond, in contrast to glass,
feels cold when you touch your skin with it. It conducts the heat out of your body very efficiently to the surroundings.
|
| |
 |
Materials Scientists and Engineers would love to use diamond for all kinds of
application. Unfortunately we cannot make nice big diamond crystals. That limits technical applications to small
artificial crystals in slurries for cutting and polishing since big natural crystals
do not come exactly cheap and are thus used almost exclusively for non-technical applications. |
 |
The diamond structure – i.e. a fcc lattice and two atoms as base –
is a much loved structure among atoms. |
|
 |
Take the blue and green spheres to be silicon (Si) or germanium (Ge) atoms and
you have the structure of those semiconductors.
Take the blue spheres to be gallium (Ga) or indium (In), and the green
spheres to be arsenic (As), phosphorous (P) or antimonide (Sb) atoms, and you have compound semiconductors like GaAs or
InP that are indispensable to optoelectronics.
The atoms are strongly bonded to
each other but not quite as strong as carbon atoms. Silicon is quite hard but not as hard as diamond. |
 |
Here is the graphite
structure: |
| |
|
|
|
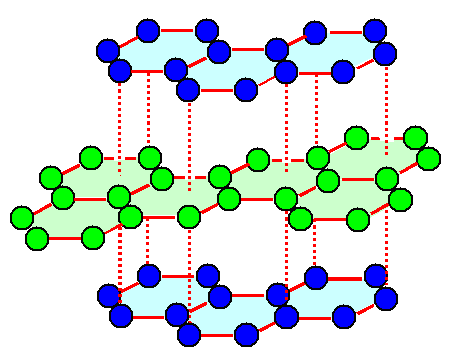
| | Graphite crystal structure |
|
| | |
|
|
 |
Blue and green spheres are carbon atoms, the full red lines are strong carbon
- carbon bonds. The dashes red lines are very weak bonds and that's why it is easy to move one atomic layer relative to
another one. |
|
 |
It is clear that we have a hexagonal lattice here. It is not so clear how the
atoms are distributed. You need in fact three carbon atoms per lattice point, and figuring out exactly how everything hangs
together is a typical not-so-easy exercise question in lectures. |
 |
Graphite once more got its names from the ancient Greek ("grapho"=to
draw, write) in 1789 when it was used in pencils. People, however, called it "lead" even so it has nothing to
do with the element lead (Pb) as we know now. People then thought that natural graphite was some lead mineral. In German,
a pencil is still called "Bleistift", literally "lead pen". |
|
 |
The English word "pencil", by
the way, has an interesting etymology, too. The word goes back to the late 14th century when it denoted an artists fine
brush (of camel hair) and is based on the old French "pincel" (pinceau in modern French), which in turn derives
form the Latin "penicillus", literary "little tail", the diminutive of "peniculus"=brush,
itself a diminutive of "penis"=yes, also tail.
Knowing that, the good old saying (or wishful thinking of
us writers) that "the pen is mightier than the sword" acquires new and interesting meanings, as do statements
like: "I still prefer my pencil to a word processor".
More about things like that in the link. |
|
|
|
 |
On a more serious note, graphite is an extremely important High-Tech material
for various reasons. Graphite electrodes, for example, can take the heat and are absolutely indispensable for "smelting"
and processing silicon and many other elements. |
| |
|
| |
New Stuff |
 |
Carbon keeps exciting scientists because new "phases" (in quotation
marks because for purists those modifications are not true phases) are coming up almost routinely by now. The most important
ones are shown below: |
| |
 |
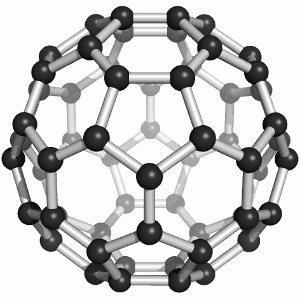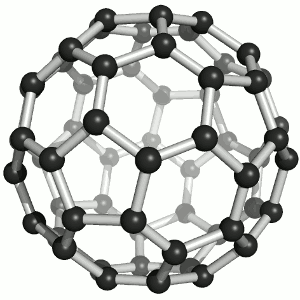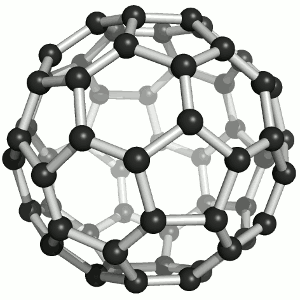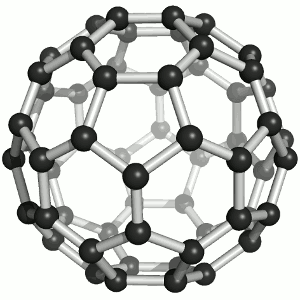
Buckminster-Fulleren, named after Richard Buckminster Fuller. He did not discover that
carbon "molecule" but was famous for his geodesic dome structures that looked like this kind of carbon.
"Bucky balls" were actually predicted in 1970 by the Japanese chemist Eiji Osawa based on theoretical calculations. He published in Japanese and was thus ignored.
Some American guys who published about that 15 years later were not ignored and got the Nobel prize; Osawa didn't.
|
| |
|
|
|
 |
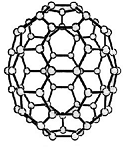 |
| Buckminster-Fullerene |
Bucky ball or C60
Yes—it mimics exactly the structure of a soccer ball, where hexagons and pentagons are sown together to form a
passable sphere. | Bucky football; one of many versions |
|
| | |
|
|
 |
Bucky balls caused a tremendous excitement in the 80ties; they were supposed to
be good for a great many applications. When extensive research and development failed to bring bucky balls to a point where
one could make serious money by selling a product and not just by writing proposals, the "new carbon modification"
field of research would have dwindled into insignificance, except: |
 |
Carbon nanotubes (CNT) were discovered. |
|
 |
The discovery of hollow, nanometer-size tubes composed of graphitic carbon is
usually credited to Sumio Iijima of NEC in 1991.
However,
already in 1952, L. V. Radushkevich and V. M. Lukyanovich published clear images of 50 nanometer diameter tubes made of
carbon in the Soviet Journal of Physical Chemistry. They wrote in Russian and were evil communists; it goes without saying
that their discovery was ignored in the West.
As it turns out, some carbon nanotubes are always around in all kinds
of stuff that contains carbon (including wootz steel swords). I bet you will find some in the black stuff that is almost
impossible to get out of your oven. You cannot find carbon nanotubes, however, if you can't see them. So the official "discovery" had to wait until powerful transmission
electron microscopes emerged. |
| |
| |
| |
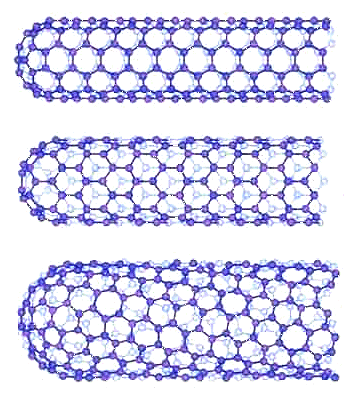 |
| Carbon Nanotubes |
| Carbon nanotubes can be "made" by rolling "chicken-wire" sheets of carbon
(now called "graphene", see below) to form a tube. You can do that in several different ways as shown above, and
the resulting tubes have completely different properties., e.g. being a metal-like conductor,
a semiconductor like silicon, or an insulator. |
|
| |
|
|
 |
It's not too difficult to calculate the different electrical properties and the
superior mechanical properties of CNT's. If you take them to be a (nano) rope, they are much "tougher" than anything
else otherwise known. In other words, they can bear much larger weights than a rope of the same
diameter made from other material, e.g. high-strength steel.
That's nice but not of much technical value
because even on an extremely strong nanorope you still can only hang nanoweights.
Nobody has quite figured out yet how to make macro ropes or other useful things out
of CNT's. |
| | |
When extensive research and development failed to bring CNT's to a point where
one could make serious money by selling a product and not just by writing proposals, this "new carbon modification"
field of research would have dwindled into insignificance, except: |
 |
Graphene was discovered. |
|
 |
Graphene is a flat but slightly undulating monolayer of carbon atoms tightly packed
into a honeycomb lattice, see below. The Nobel Prize in Physics for 2010 was awarded to Andre
Geim and Konstantin Novoselov who made the stuff
in 2004 by exfoliating it from a graphite crystal with Scotch tape. Never before was a Noble prize won by such a simple
experiment.
Here is a schematic figure of what it looks like: |
|
| |
| |
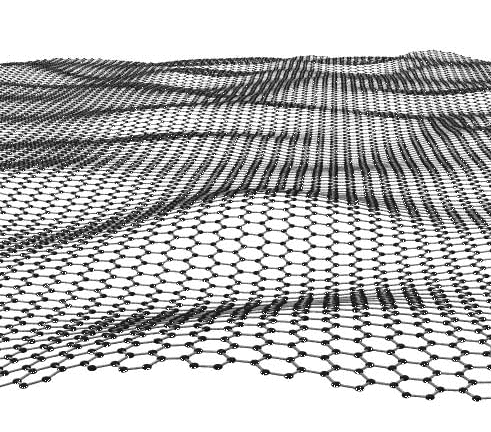 |
| Graphene |
|
| | |
|
| |
 |
Graphen is extensively researched right now because it shows great promise for
exciting new applications....
(To be continued) |
| | |
© H. Föll (Iron, Steel and Swords script)





