|
Glide Systems and Ease of Deformation |
|
Note: this module is pretty much self-contained and
understandable in the context of what is explained in the backbone. Nevertheless, it is advantageous to give the two science
modules listed a quick look, too. |
|
 |
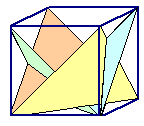
If one dislocation moves through a crystal, it shears one part with the respect
to the other part a tiny little bit. Many dislocations of the same type moving the same way just make the shear larger.
This is shown on the left-hand side of the figure below.
You can only produce very specific deformations
this way. |
|
 |
No, you can't just have your dislocations move some other way. In real crystals
there is a limited number of planes on which dislocations can move, and a limited number
of shear directions (called "Burgers vector" for reason
that need not concern us here) they can produce. The two limits come straight from the fact that Burgers vectors must be
the shortest possible translation vectors of the lattice, and that glide planes should be the ones with the highest packing density of atoms.
So let's pick
a second set of an allowed plane (called "glide plane") and move a dislocation there in an allowed shear direction.
This is shown on the right-hand side of the figure below. | |
|
|
 |
Considering that the steps produced by moving a dislocation are very small, what kinds of
shapes can we make this way? This is not an easy question. The really tough question, however, is:
How many independent
combinations of glide planes / Burgers vector or, as we going to call that "glide systems",
do you need to be able to produce any shape?
If you know and enjoyed "The
Hitchhiker's Guide to the Galaxy" from Douglas Adams, your
guess probably would be "42". Well, you are wrong. The proper answer is "5". Sorry about that but the question to the answer "42" is still not known.
All material scientists know
that the proper number is 5 but very few know how it is derived. It is a bit like the 14 Bravais
lattices. | |
|
 |
Just five glide systems are enough to produce
any kind of shape from your original body by running a sufficient number of dislocations through it.
|
| |
| |
| |
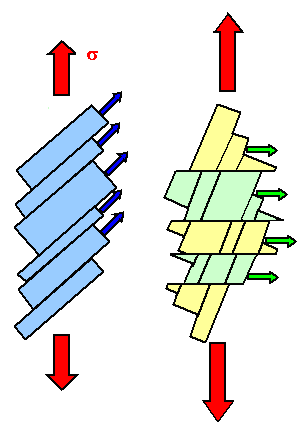 |
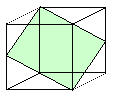
| Deformations by dislocation movement on one or two glide systems. |
|
| |
|
|
 |
You can change the original rod into many shapes by operating one or two glides
systems - but not into all possible shapes. For that you need five glide systems |
 |
So how many glide system do we have in the more important Bravais
lattices / crystals? My students always find this a very hard exercise to do, even so it only contains "elementary"
geometry. Anyway, here is the answer.
(Ignore the Miller indices
given for the lattice planes if you are not acquainted with them and even if you are, don't worry about the 4-indices system
for the hexagonal crystal). |
| | |
|
| |
Densely packed planes in fcc, bcc and hex lattices
or the major glide planes for dislocations
in those crystals.
Some prominent crystals using specific glide planes are indicated. |
| fcc | bcc |
hcp |
{111}
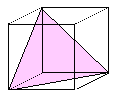
Al, Ag, Cu, Ni, g- Fe,
.. | {110}
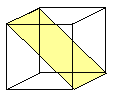
a- Fe, W, Mo, b-brass |
{0001}
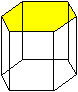
Cd, Zn, Mg, Be, Al2O3 |
There are four {111}-type planes | {211}
a- Fe, W, Mo, Na |
{1,0,–1,0}
Ti, Zr |
{321}
a- Fe, K
| {1,0,–1,1}
Ti, Mg (rarely) |
| {111} most dense. No other planes come close |
{110} most dense but the others are quite similar |
{0001} most dense but others are close. |
| All Burgers vectors are a/2<110> type. |
All Burgers vectors are a/2<111> type. |
Shortest Burgers vector is a/3<1,1,–2,0>. Others are possible. |
| Number of crystallographically identical planes: |
{111}: 4
(111), (-111),
(1-11), (-1-11) |
{110}: 6
{211}: 12
{321}: 24 |
{0001}: 1
{10–10}: 3
{10–11}: 6
|
| Number of different Burges vectors scontains in a plane |
{111}: 4
(111), (-111),
(1-11), (-1-11) |
{110}: 2
{211}: 1
{321}: 1 |
{0001}: 3
{10–10}: 1
{10–11}: 1 |
| Number of glide systems = combinations plane / Burgers vector |
| 12 (= 3 · 4) |
{110}: 12 = 6 · 2
{211}: 12 = 12 · 1
{321}: 24
= 24 · 1
|
{0001}: 3 = 1 · 3
{1,0,–1,0}: 3 = 3 · 1
{1,0,–1,1}: 6 = 6 · 1 |
|
| |
|
 |
So we have enough glide systems for fcc and bcc crystals. In hexagonal systems
things are more complex. The easy basal or {0001} plane supports only 3 shortest Burger vectors. Deforming an hexagonal
crystal thus needs to activate one or both of the other glide planes and unfavorable Burgers vectors, which is possible
but more difficult. It follows that hexagonal crystals must be less ductile and more brittle then their cousins, which they
are, indeed. That is one of the reason why hexagonal magnesium (Mg), a metal offering an extremely good strength-to-weight
ratio, is not (yet) used as much as it should be. |
|
 |
Note that the whole business of glide systems in bcc crystals
is more tricky then for fcc crystals. While there are more glides systems, none of them is as easy to operate as the {111}/<110>
system of the fcc crystals. In comparison to fcc crystals, this tends to make bcc crystals tougher to deform, somewhat more brittle, and in particular given to "cold shortness", or becoming completely brittle at low temperatures.
Note too that our beloved iron uses all possibilities offered, in contrast to other
bcc crystals that have some favorite glide systems. That may help the smith when he bangs it into shape but doesn't make
the science of deforming iron much easier. |
| | |
|
© H. Föll (Iron, Steel and Swords script)








