| |
|
| |
What are Gemstones? |
 |
A few of the many crystals we find in nature are called "gemstones", and most of
them are single crystals. But not every single
crystal found in nature is a gemstone. We like to have the following properties for gemstones:
- Rarity. Things that aren't rare or exclusive but common are never considered noble.
Neither feldspar, rock crystals or fluorite crystals are gemstones, nor the rather pretty pyrite or fools gold crystals.
They are just not rare enough.
- Perfection. It's not enough to be a single crystal, it is important to be as clear
and flawless as possible. It is not important in this context that the crystal be free of lattice defects and impurities,
it only must appear clear to the eye. A few foreign atoms might even be required to turn an otherwise worthless crystal
into a gemstone, as we shall see below.
- Color. Rubies are red, sapphires are blue,
citrins are yellow, amethysts are violet, and so on. In all of
these (and many other) cases the actual pure crystal is colorless and not a gemstone. For attaining color it needs to contain
certain impurity atoms that are dissolved in the crystal lattice.
- Hardness. Hard crystals are somehow more noble than soft ones, even so women normally
go for the soft things (cashmere wool is better than regular wool, ...). Why? Ask your psychologist.
- Size counts - at least as far as hard gemstones are concerned. A diamond of 100
carat (1 carat=0.2 g) is far more valuable than 100 stones with 1 carat each.
- Finally, the way the stone is cut is of importance. Up to the late 16th century
all gemstone "cuts" were cabochon, with a smoothly
curved surface; just look at old crowns and reliquaries or the picture below.
The facetted cut we have today was invented
by Jules Cardinal
Mazarin (1602 - 1661). Those were the good old
times when men (including men like the Cardinal who were not allowed to exercise their manliness 2))
had no problem to dress elaborately and show their wealth. |
| |
|
| |

|
Cabuchon cut gem stones in the sail of the "Golden Ship"
(12th century) in Uelzen / Germany |
|
| | |
 |
It was, and to some extent still is, common practice to distinguish between the "real"
or precious gemstones (essentially diamonds, rubies, sapphires and emerald) and semi-precious stones—the rest. |
|
 |
Nowadays some of us live in a democracy, and we don't discriminate against half-breeds (of
the mineral variety) anymore. We just have gemstones or jewels now, including "stones" that aren't true crystals,
for example pearls or amber, rather tricky and unusual crystals like opals, or all kinds of things not found in nature but
made by man (e.g. zirconia). |
|
 |
Here I only distinguish between crystalline and non-crystalline jewels. As far as the crystalline
stones are concerned, they come in a few basic structures that we will give a first quick look. |
| | | |
 |
Corund(Ruby and Sapphire) |
|  |
Corund is an an Indian word for the humble but rather hard aluminum oxide (Al2O3)
we use in polishing pastes, on emery paper and, in single-crystal form as watch glasses and substrates for microelectronic
circuits. For the "cheap" applications, Al2O3 "contaminated" with iron (Fe) and
looking dirty is used. Its ("trigonal")
crystal lattice is a bit more complicated that that of diamond, see below. |
|
 |
Now "contaminate" otherwise good single crystals with a little bit of chromium (Cr)
or titanium (Ti), and you get very valuable "rubies" or "sapphires",
respectively. Sapphires usually are blue, but they can have all kinds of colors, from yellow to violet, depending on what
kind of defect, exactly, sits in the lattice. Of course, the natural rubies and sapphires were contaminated by Mother nature.
|
| |
But some of us can also make artificial rubies and so on; and artificial
or synthetic ruby was the heart of the very first Laser in 1960. Note also that many (detrimental) defects in natural gemstones
can be "healed" by simply heating to a high temperature, causing some question about where, exactly, you find
the dividing line between "naturally perfect gemstone"=expensive and "artificial gemstone"=cheap. |
| | |
| |
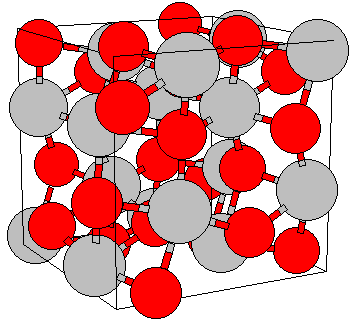
|
 |
| Corund lattice, red=oxygen (O), grey=aluminum (Al) |
The lattice is often (and correctly) described as hexagonal, but the trigonal or rhombohedral
cell given above is simpler. It is not as complicated as it looks - it's just a stacking of O2– octahedra
with Al3+ inside as shown on the right.
|
| Source: adopted from http://cst-www.nrl.navy.mil/lattice/struk/d5_1.html
and Wikipedia |
 |
 |
| Raw and cut sapphire. |
 |
 |
 | Raw and cut ruby
Rubies / diamonds pendant |
|
| | |
|
|
 |
Good rubies are just as expensive as diamonds; sapphires tend to be a bit cheaper.
Lesser stones can be rather cheap, however. Both gems had technical uses, e.g. the "ruby" bearings in watches
or the "sapphire" pick-ups in old-fashioned record players. |
 |
Beryl |
|
 |
The name Beryl is derived (via Latin: beryllus, Old French: beryl, and Middle English: beril)
from Greek beryllos, which referred to a "precious blue-green color-of-sea-water stone". It goes even farther
back, ultimately to Sanskrit. The Latin word "berillus" was abbreviated to brill, a word root we find in the Italian
"brillare" meaning shine or "brilliant", meaning diamond (and shining) in English and German. The French
word "brille" and the English word brilliance means shine or shining once more, and the German "Brille"
means eye glasses. |
|
 |
The oldest "Brillen"=eye glasses, one of the key inventions of humankind, were made
from beryl that was ground to lens shape. I'm tempted to claim that it was a German invention but something akin to the
glasses you wear in front of your eyes was first made and used in Italy, in the 11th century. |
| | | |
| |
 |
| Beryl varieties |
| Source: Photographed in the Metropolitan Museum NYC. |
|
| | | |
 |
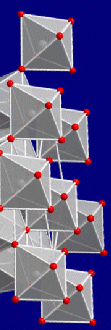
Beryl is basically a beryllium - aluminium silicate with the basic composition Be3Al2(SiO3)6.
The crystal structure is a bit involved. Essentially, Si6O18 rings as shown on
the right-hand side in the figure below, are stacked on top of each other, with the metal-oxygen units "in between". |
| | | |
| |
 |
Layer of a beryl crystal lattice
|
| Source: partially from wikipedia |
|
| | |
|
 |
Beryl is the base for a number of gem stones. Once more, the kind of impurity contained in
solution in the crystal defines its color. |
|
 |
Emerald, the fourth of the four kinds of "precious"
stones of old is green because the beryl contains a bit of chromium (Cr), or more precisely, chromium tri-oxide (CrO3) |
|
 |
Aquamarine, or spinel, is light blue to greenish.
The reason is probably iron, or more precisely Fe2+ in, for example FeO. Fe3+, for example in Fe2O3,
produces a golden-yellow color (see below), and when both Fe2+ and Fe3+ are present, the color is
a darker blue. |
|
 |
Morganite, or "pink beryl", "rose
beryl", "pink emerald", and so on, is a rare light pink to rose-colored variety of beryl. Its color derives
from manganese (Mn). |
|
 |
Golden beryl and heliodor
range in color from pale yellow to a brilliant gold. Golden beryl refers to pure yellow or golden yellow shades, while heliodor
refers to the greenish-yellow shades. The golden yellow color is attributed to Fe3+ ions. |
|  |
There are more varieties. The periodic table, as you know, has a a lot of "dirt"
left to put into the beryl lattice. |
| | |
|
| |
 |
| Natural and cut emerald |
 |
 |
| Natural aquamarine | Natural morganite |
 |
Golden beryl
Flawless 2054 carat stone on display in the Hall of Gems, Washington,
D.C. | | Source: (wikipedia) |
|
 |
Quartz
Quartz or silicon dioxide (SiO2) is one of
the most important technical materials. Suffice it to say that there would be no electronic or optical industry without
SiO2. Whenever you look out through a window you're looking through ("dirty") silicon dioxide called
glass.
Silicon dioxide is also the base material for a long list of gemstones. |
|
 |
Rock crystal, the pure (trigonal)
naturally occurring SiO2or quartz crystal. Rather large, completely transparent and colorless crystals can be
found. They were used to cut all kinds of figures, bowls, vessels, etc. for the treasure vaults of the rich.
Synthetic
quartz single crystals are important for the "quartz oscillators" inside digital watches, cell phones and most
other electronics. |
| |
|
| | |
 |
| Rock Crystal lattice (one plane) |
 |
 |
Rock crystals and goblet cut from rock crystals
|
| Source: Goblet photographed in: "Landesmuseum Stuttgar", Germany |
 |
| Relatively rare quartz "bipyramids" from Brilon, Germany |
| Source: Photographed in the Brilon town museum |
|
| | | |
|
 |
Amethyst
Up to the 18th century, before the discovery of
large amounts of good amethyst crystals in Brazil, amethyst was counted among the really precious stones. Several great
properties were attributed to amethyst. Its name comes from Greek, where the word "amethystos" meant: "not
drunken". Amethyst was considered to be a strong antidote against drunkenness, which is why wine goblets were often
carved from it. It was also supposed to help wound healing, and to prevent theft.
Well - it's just common rock crystal
with ionized iron in it. The ionization of the iron "dirt" is crucial, it might have resulted from irradiations
by natural radioactivity in the course of a few million years. |
|
| |
|
|
 |
| Collection of Amethysts Geodes and Detail |
|
| | | |
|
 |
Rose quartz
is the pink to rose-red variety of quartz. The color is due to trace amounts of titanium, iron or manganese, and to
more complex inclusions. Rose quartz is typically not clear but at best translucent.
In transparent form (rarely found)
it is called pink quartz and its color is thought to be caused by trace amounts of phosphate
or aluminium. The color in these crystals is apparently photosensitive and subject to fading. The first crystals were discovered
in a pegmatite found near Rumford, Maine, USA, but most crystals on the market now come from Minas Gerais, Brazil.
|
 |
Then we have smoky quartz, gold-yellow citrine
as single crystals, and Chalcedony, Agate, Onyx,
Jasper, and plenty more polycrystalline or amorphous ("glassy") stuff. |
| |
 |
Of fleeting interest for us is chalcedony. It is an intergrowth of extremely fine crystals of normal quartz
and a variety with a different lattice structure called moganite. Since it is almost amorphous it looks glassy or waxy.
Ad a little dirt and you get a whole range of minor gemstones like agate, aventurine, heliotrope, onyx and orange-red carnelian.
Carnelian was used quite a bit in antiquity, like in the pectoral below, but
also as decoration in one of the earliest iron swords. |
| | |
|
| | |
 |
Pectoral of Princess Sit-Hathor Yunet, the daughter of
Pharaoh Senusret II (1897 BC to 1878 BC)
Cloisonné inlays on gold of carnelian, feldspar,
garnet, turquoise, lapis
lazuli. | | Source: Metropolitan Museum, NYC |
|
| | | |
|
But I'm not going to dwell on all those many SiO2 forms anymore but turn
to one of the most amazing quartz-based gemstone: |
|
 |
Opal
Opals are crystals (fcc cubic) - but not of atoms
but of small amorphous quartz spheres or glass beads with diameters around 200 nm; about 100 times smaller than the diameter
of a hair. If you think that's so small that it doesn't matter relative to atoms, think again. A 200 nm sphere contains
very roughly 300.000.000 atoms.
The lattice
constant of opals thus corresponds to the wave length of visible light and that causes its spectacular optical properties.
Nowadays we call structures like that of opals "photonic
crystals". Synthetically made photonic crystals caused a great stir in the scientific community in the 90ties
of the old century and are still pursued for various "high-tech" optics applications. |
| | | |
| |
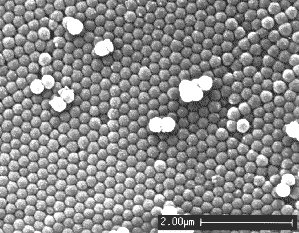 |
 |
SEM picture of opal showing the
"glass" bead structure |
Opal |
|
| | | |
 |
Garnets
Garnets are crystals with a common basic structure
of the type C3A2D3O12 or C3A2(DO4)3
with:
- C: doubly positively charged metal ion, surrounded by 8 oxygen ions,
for example Mg2+, Ca2+, Fe2+, Mn2+.
- A: metal ions with three or four positive
charges, surrounded by 6 oxygen ions. Examples are Al3+, Cr3+, Fe3+.
- D: mostly ions with 4 negative charges, surrounded by 4 oxygen ions, e.g. Si4+,
Al3+, Ga3+, Fe3+.
|
|  |
"Garnet" derives either from Middle English
"gernet"=dark red, or from Latin "granatus"=grain;
possibly referring to the red pomegranate seeds. |
|
 |
Considering that garnets consists of at least 4 different atoms, their crystal structure tends
to be a bit complex. Essentially, you are stacking all those tetrahedra, octahedra, and other "edra" hinted at
above; see also the corund example. The general shape is essentially cubic. |
| | |
| |
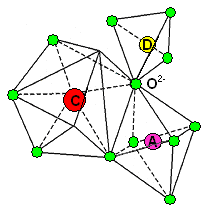 |
 |
| The "edra" of the garnet structure (Green=oxygen) |
| Basic Garnet crystal lattice |
The figure on the right shows "Pyrope" with C=Mg2+
(red); A=Al3+ (violet);
D=Si4+ (yellow). The oxygen ions are light blue.
|
| Source: Adopted from a University of Salzburg page |
|
| | |
 |
It is clear that we find a tremendous variety of garnets out there. If they come in nice colors
and shapes, they are gemstones.
Some garnets have been used extensively in old times. Not only as a gemstones but also
as abrasive. Some major varieties are |
| |
 |
Almandine, Fe3Al2(SiO4)3
I can't describe it better than wikipedia: "Almandine, sometimes incorrectly called almandite, is the modern gem
known as carbuncle (though originally almost any red gemstone was known by this name). The
term "carbuncle" is derived from the Latin meaning "live coal" or burning charcoal.
The name Almandine
is a corruption of Alabanda, a region in Asia Minor where these stones were cut in ancient times.
Chemically, almandine
is an iron-aluminium garnet with the formula Fe3Al2(SiO4)3; the deep red transparent
stones are often called precious garnet and are used as gemstones (being the most common of the gem garnets).
Almandine
occurs in metamorphic rocks like mica schists, associated with minerals such as staurolite, kyanite, andalusite, and others.
Almandine has nicknames of Oriental garnet, almandine ruby, and carbuncle." Thanks, wiki!
Red garnets were the
most commonly used gemstones in the Late Antique Roman world, and the Migration Period art of the "barbarian" peoples who took over the territory of the Western Empire.
They were often inlaid in gold cells in the cloisonné technique, a style often just called garnet cloisonné,
found from Anglo-Saxon England to the Black Sea. |
| |
| |
|
|
 |
Almandine Garnets in Modern Reproduction of 6th Century Sword
The real thing |
| From the pages of swordsmith Patrick Bárta |
|
| | | |
|
 |
The rest pales in comparison. We have
- Pyrope, Mg3Al2(SiO4)3, with
magnesium (Mg) instead of iron (Fe) compared to almandine. It's also known as Bohemian garnet from the Czech Republic, and
was and is used as gemstone.
-
Spessartine, Mn3Al2(SiO4)3,
with manganese taking first place now. It's found in the German "Spessart" and comes red to yellow.
- And so on. You get the drift.
|
| |
 |
 |
| Garnets | Almandine
From Wikipedia |
| If you find some "obvious" garnets like those shown in the picture above on the left,
it is not all that obvious, which kind exactly they are. |
|
| | | |
 |
|
There are plenty more crystalline gem stones, for example spinel, tourmaline, Rhodonite, ...
But now let's look at some of the poly-crystalline or amorphous stuff. |
| |
| |
|
Poly-crystalline
gemstones |
 |
We have
- Light blue to greenish turquoise or "Turkey stone" ((CuAl6[(OH)8[(PO4)4]·4H2O);
a copper bearing mineral.
- Dark blue lapis lazuli
((Na, Ca)8|(SO4, S, Cl)2|(AlSiO4)6]) or lazurite
(the "stone of Lazhward"). Lapis was treasured throughout the antique word not only as gem stone but also as a
base for (very expensive) blue paint (the ultramarine pigment).
- Greenish Jade, coming in several variants based on NaAl(Si2O6).
Jade was highly treasured in the Chinese empire from very early until recently.
|
| | |
| |
 |
| Jade Figure; early 1900 century |
| Source: Photographed in the Metropolitan Museum NYC. |
|
| | |
| |
Amorphous an
Biological gem "stones" |
 |
Essentially we have: |
|
 |
Glass. Of course, fake gemstones are made from glass and that's not what I mean. But
cheap glass in all colors as we know it wasn't always around in quantity. The ancients treasured glass things (and used
it to fake real gemstones, too). The stained glass windows of old cathedrals were true treasures then and now. Their color
derived in part from some nanotechnology (look it up yourself).
Obsidian, a natural black glass of volcanic origin was used more for making tools then for
jewelry, just like its more common relative, the flint stone. |
|
 |
Amber, or petrified tree resin. Petrification happens if no oxygen
is available (i.e. under water) and if there is enough time like 400 Mio years. Occasionally insects were caught in the
resin and then preserved for almost eternity.
Amber is a polymer consisting of 73,8 % carbon (C), 9,5 %
hydrogen (H), 10,5 % oxygen (O) and und 0,1 % sulfur (S). It is found in quantities right where I live: along
the shore of the Baltic Sea.
Amber is not very precious today but still much in use as gemstone. Already in the stone
age it was treasured enough to induce trading over large distances . |
| |
| |
|
|
 |
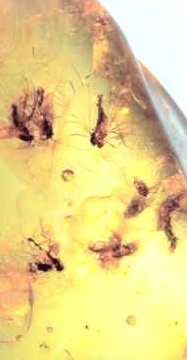 |
| Jade Buddha |
Amber with enclosed
400 Mio year old flies |
|
| | |
|  |
Pearls, shells of slimy animals likes snails or clams, and other
"petrified" mollusk snot. Pearls are made whenever mollusks like oysters put the stuff around alien objects in
their body (e.g. sand grains) that they can't get rid off. Pearls consist of nacre or "mother-of-pearl"), something we will encounter
in the backbone of the Hyperscript.
The luster of pearls is once more a "photonic crystal" effect as in the case of opals and "pearlite" a (pseudo) phase of steel..
Perfect round pearls used to be just as
expensive as diamonds throughout most of history. They only became cheap after seeded pearls conquered the market. So when
you encounter pearls in old jewelry or just in old picture, they heralded great wealth. |
|  |
We will encounter nacre as a model material for steel,
and we will encounter"pearlite", as a (pseudo) phase of steel, so pearls are
very meaningful for sword lore. |
| |
|
| |

|
 | | A pearl in situ |
Jet mourning brooch |
|
| | | |
|
 |
Jet or gagat is "petrified"
jet-black coal. Considering that coal is sort of petrified wood, that is a lot of petrification.
Jet was fashionable
on and off, its high point was in the Victorian era. It was never considered to be very precious and sometimes worn as "mourning
jewelry". Look a the brooch above and you will feel properly depressed. |
| | | |
|
| |
| |
1) "Diamonds Are
a Girl's Best Friend" is a song first introduced by Carol Channing in the original Broadway production of "Gentlemen
Prefer Blondes" (1949), which was written by Jule Styne. Marilyn
Monroe did the song in the 1953 film version of "Gentlemen Prefer Blondes" and made it famous.
The song was listed as the 12th most important movie song of all time by the American Film Institute.
Here is the text: |
| |
The French are glad to die for love.
They delight in fighting duels.
But I prefer a man who lives
And gives expensive jewels.
A kiss on the hand
May be quite continental,
But diamonds are a girl's best friend.
A kiss may be grand
But it won't pay the rental
On your humble flat
Or help you at the automat.
Men grow cold
As girls grow old,
And we all lose our charms in the end. |
There may come a time
When a hard-boiled employer
Thinks you're awful
nice,
But get that ice or else no dice.
He's your guy
When stocks are high,
But beware when they start to descend.
It's then that those louses
Go back to their spouses.
Diamonds are a girl's best friend.
I've heard of affairs
That are strictly platonic,
But diamonds are a girl's best friend. |
| | |
 |
| Marilyn Monroe in "Gentlemen Prefer Blondes" |
| | |
|
But square-cut or pear-shaped,
These rocks don't lose their shape.
Diamonds
are a girl's best friend.
Tiffany's!
Cartier!
Black Starr!
Frost Gorham!
Talk to me Harry Winston.
Tell me all about
it!
There may come a time
When a lass needs a lawyer,
But diamonds are a girl's best friend.
|
And I think affairs
That you must keep liaisonic
Are better bets
If little pets get big baguettes.
Time rolls on,
And youth is gone,
And you can't straighten up when you bend.
But stiff back
Or stiff knees,
You stand straight at Tiffany's.
Diamonds! Diamonds!
I don't mean rhinestones!
But diamonds are a girl's best friend. |
|
| |
© H. Föll (Iron, Steel and Swords script)




































