|
8.4 Be Cool! |
| 8.4.1 Martensite |
| |
Surprise! |
 |
It's clear by now that most of the time the crystal wants to do the opposite of
what you want it to do. The crystal wants big grains, large precipitates, and few dislocations - in other words, it wants
to be a softy.
For sword blades we want the crystal to be hard and tough. Nothing
helps but to interfere massively with its preferred life style. The way to do that is to be cool1). And to get your steel to be that too - and presto! |
|
 |
We know that the first strategy for keeping the crystal from doing its thing is not allowing it enough time for moving its
atoms around.
In other words: we must quench the crystal, we cool it down from
some high temperature real fast. If we do that from a temperature above the austenite
transition, the crystal has very little time for rearranging its own atoms from fcc austenite to bcc ferrite and in particular
for getting most carbon atoms out of the newly formed ferrite in order to make cementite.
If the time is simply too
short to produce pearlite and whatever else is called for by the phase
diagram, new things must happen. |
|
 |
Let's look at the extreme: If you could cool down arbitrarily fast, atoms can't
move at all and you would find the same structure at room temperature that you had at high temperatures.
In the laboratory
or in special factories we can do this under special circumstances for special geometries. We can cool a thin
piece of any metal down from above 1000 K to room temperature in a fraction of a second. What we get are thin ribbons of
amorphous metal that are quite useful for example when it comes to magnetic
properties.
Many metals then "freeze-in" the liquid structure they had at the high temperature and are
amorphous solids with peculiar properties. The process is called "melt spinning"
and you can look it up if you want. | |
'' |
| |
When we cool down extremely quickly we do not get the nirvana state as called
for by the phase diagram but a metastable phase
that tries as hard as it can to change to the stable one. Since this takes some energy, metastable phases—like diamond—can live very long at room temperature.
However, as soon as you heat it up to a sufficiently high temperature it will transform to the stable phase. |
|
 |
Melt spinning of iron or steel does not produce anything even remotely thick enough
to be useful as sword blade. The best you could expect for a real sword blade is to
quench it so fast that parts of the austenite structure are "quenched-in",
at least in the outer parts of the blade that cool down the fastest. That is the best you can expect with what you have
learned so far. If that expected structure would be good for sword blades is not yet clear, however. |
 |
Surprise! |
| |
Something unexpected will happen!
|
|
|
 |
The "best" you and everybody else could reasonably expect
does not happen! Plain carbon steel does not freeze into the austenite structure, no
matter how fast we cool it down. Plain carbon steel still has a big trick up its sleeve. If we cool it real fast, it produces
something completely new, a kind of metastable phase, that we call martensite,
after Adolf Martens (1850
- 1914).
Martensite formation is rather strange. It does not happen gradually while we quench. It happens all
of a sudden at temperatures as low as just a few 100 centigrade when single atoms
can't really move much anymore. But dislocations still can, and martensite formation
is a process that involves lots of dislocations. |
 |
Let's look again at the driving force business before we proceed with martensite formation. Let's look at you (and me) for that. You
and I are supposed to be good and law-abiding citizens. I'm sure that you strive all the time to achieve the supreme state
of being a good, responsible, law abiding, and so on citizen, who reads educational stuff like this article, instead of
guzzling beer and munching extremely unhealthy food.while watching scantily clad females on TV doing things that your local
moral authority would not approve of. |
|
 |
I also do some of that on occasion. Especially whenever the need to be particular
good is not so pressing because, for example, I still fit into my clothes or my wife is not around.
However, if the
deviation from what I should be weight-wise (for crystals it would be nirvana-wise) becomes larger, my attempts to achieve
the proper state of being become more severe. Shortly before I would explode, I will take recourse to desperate measures
and drink water instead of beer. |
 |
We are talking driving forces here
once more. What's weight difference for me, is energy difference for crystals. |
|
 |
If you are still in the austenite state and it is just a little
bit below the transformation
temperature, things are not serious yet. The difference in energy between ferrite and austenite is small.
But being
still in the austenite state a lot below the transformation temperature is really, really
bad. Ferrite now would have a much lower energy than austenite. This state of being cannot be tolerated anymore.
The
need to turn from an fcc austenite crystal into a bcc ferrite crystal gets absolutely peremptory. All your atoms are involved
in this because they all need to change their surroundings from 12 next
neighbors in the fcc austenite lattice to 8 in the bcc ferrite lattice.
In addition, you also would like to get
rid of the carbon in the ferrite or a phase. Ferrite atoms just do not like to have a carbon
neighbor. Getting rid of the carbon is a bit less pressing, however, since it concerns only those of your iron atoms that
are next to an interstitial carbon atom whereas the need to be ferrite concerns all.
Getting rid of surplus carbon would
mean to move individual carbon atoms from wherever they are to somewhere else. But individual atoms can't be moved in a
civilian way by diffusion anymore, so you
simply cannot make carbon free ferrite grains, period.
The only
two options left are to remain (metastable) austenite or to go for martensite as a compromise.
It's a lousy thing to do but better than to remain austenite. |
 |
So, what exactly is martensite? A rather special
microstructure or arrangement of iron atoms! Even with our present knowledge of what
crystals are and do, fortified with a phalanx of heavy computers, I doubt that we would have predicted martensite formation.
It seems that crystals are still smarter than we are when driven to extremes.
Martensite formation also occurs in other
metals and it is a pretty queer thing there too. |
|
 |
When people like Adolf Martens
saw martensite in their optical microscopes, they could not know what they saw and how exactly that stuff had formed. But
they could describe it and give it a name. They also figured out that the martensite
in steel was extremely hard and thus useful. |
|
 |
So once more, what is martensite?
|
| |
Martensite is a distorted bcc ferrite
lattice in parts of a steel crystal
that still contains dissolved carbon
and is full of defects
|
|
| |
- "Distorted" means that instead of a perfect cube you have a (slightly)
elongated cube (or a "tetragonal"
structure).
- "In parts" means that particles of the metastable martensite phase are
embedded in something else. There is no such thing as 100 % martensite. The something else could be regular ferrite / pearlite
/ bainite (wait) or even some retained austenite.
- "Still containing dissolved carbon" means that there is far more atomically
dissolved carbon in the martensite then would be proper for stable ferrite. That's why the lattice is distorted.
- "Full of defects" means that there are lots of dislocations inside the
martensite, for reasons we will see shortly.
The hardness of martensite comes from the defects it contains,
in particular from the dislocations. Hypothetical defect-free martensite would be simply ferrite and that is not very hard.
|
| |
| |
 |
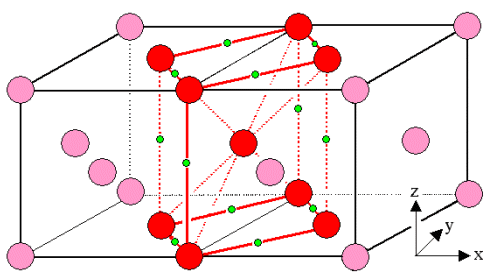
Bain realized that the face-centered close-packed austenite crystal (with the
occasional carbon atom sitting in one of the green positions indicated) actually contains a distorted
body-centered lattice as marked by the red spheres in the figure above. |
|
 |
It's a perfectly rectangular lattice but not a cube since one side is longer than the other two (that makes it tetragonal). Your halfway decent
general education should enable you to find out the precise dimensions of the red cuboid.
All that the fcc austenite needs to do for turning into a bcc ferrite crystal is to expand
a little bit laterally (x and y direction) and shrink a bit vertically (z direction).
Doing that would be a great way to satisfy that urgent desire to become bcc. But how could one do such a transformation
all over the crystal in not just one elementary cell of the crystal but in many connected ones? For doing this not only
do the atoms need to move, they all need to move precise amounts at the same time. |
 |
Even if we ignore the carbon for a moment, which shouldn't be there after turning
fcc austenite to bcc ferrite, the only way of doing this Bain
transformation is to move a lot of atoms in lockstep, sort of in military fashion. |
|
 |
The regular way, given time, would have been to move the atoms one by one, each one running
around pretty much at random, or in a civilian
fashion.
I didn't just make up those words by the way; they are well known in metal engineering and I have introduced
them already some time ago. |
 |
If you, the crystal, now start to entertain thoughts of " transformation
by military movement", you run into major problems (as always when asking the military in tough times to solve a problem):
- Even if you move your atoms in military fashion, you must start somewhere. Some
nucleation is required
and that is mostly difficult, as we know now. It is not very difficult for the iron in this case, because you just use whatever
defects happen to be around. In real life you just invent some atrocity perpetrated by the enemy (look up "Lusitana"
or "Gulf of Tonkin incident") or let the enemy do its worst (Pearl harbor). If all else fails you fake an attack
("Gleiwitz incident").
- If you take the red cuboid in the figure above as a nuclei for the Bain transformation, and make it into a real cube
(easy to do in your brain), it is obvious that it just doesn't fit into the remaining lattice anymore. If you then move
the boundaries between the new and small bcc part and the fcc austenite (you have seen
one!) outward into the fcc part (making the martensite grow), the newly created martensite will simply not fit properly
into the volume inside the austenite that its atoms occupied before the transformation. You have to press and squeeze like
hell to make it fit. The second problem therefore is that a lot of stress
will be created by a Bain transformation. Since you attempt the transformation in order to gain
energy, and since stress means to put up energy, this is not good.
- The third problem comes from the fact that so far the only way to move atoms in
a military fashion is by moving a dislocation through the
crystal. If you try to do this, you sooner or later realize that dislocation movements always
produce a shear deformation. The Bain transformation, in contrast
requires "push / pull" or, as we call it, a normal deformation. The differences
are made clear in the figure below; you might also want to use this
link
. |
|
|
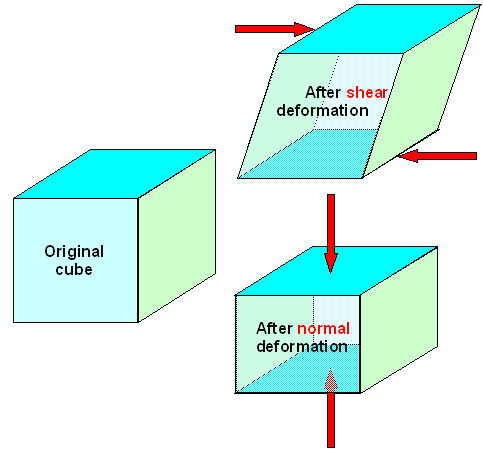 |
| Normal and shear deformations |
|
|
 |
There is a little theorem going with this: Normal deformations preserve the anglesbut
change the volume. A cube turns into a cuboid. Shear deformations preserves
the volume but not the angles. A right-angled cuboid turns into something crooked, for example.
|
 |
The long and short of this is: The Bain transformation
as envisioned is a bit too simple minded. Nevertheless, it shows the general direction of what is going on. All we need
to do is to figure out how to produce something akin to the Bain transformation by shear and not by "pulling and pushing".
|
|
 |
Luckily, a nice little theorem in more advanced math tells (some of) us that every deformation
you can produce in one way, e.g. by pure push / pull, can also be produced in the alternative way (pure shear) and vice
verse. All you need to do is a proper "coordinate transformation". That involves mathematical things far worse
than vectors so we sure don't want to go into this.
Let's only note:
- Understanding what happens when martensite formation occurs is best visualized by
the Bain model above, employing normal deformations.
- Understanding how it happens is best visualized with some (very schematic) drawings
showing shear deformations.
- The synthesis of both views is easy - in "tensor math".
|
|
 |
You sure don't want to go into some of the more sensible questions either; I just list them
to give you an idea of the complexity of the issue:
- Are there other almost bcc type lattices hidden inside the fcc lattice that can be made into proper bcc "cubes"
by some (shear) deformation?
- If there are, will there be a "bad fit" problem leading to stress?
- If there is a stress problem, can I do something to mitigate it?
|
 |
It's not possible for us to work that out for iron (and plenty of other
cases) without a bit of advanced math, so I won't even try.
Iron, however, found out how to form martensite without
math. In what follows I will try to give you an idea of how its done. There is, however, no way to make proper three-dimensional
drawings of what is really going on; schematic illustrations in two dimension must suffice: |
| |
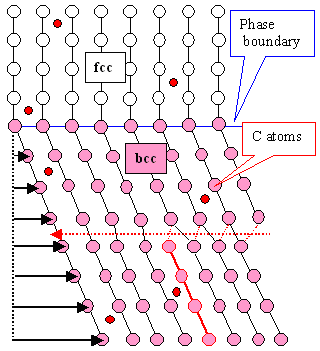 |
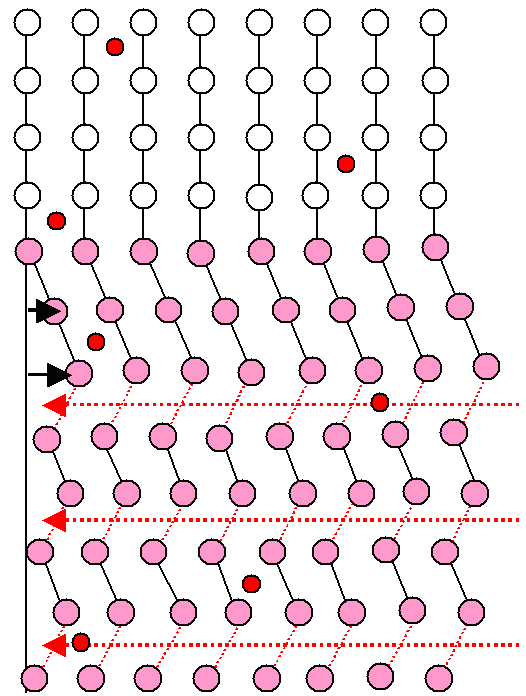 |
Schematic drawing of how martensite ( pink bcc lattice) forms
from fcc austenite (white)
by a shear process.
Also shown is a dislocation moving through the martensite. |
Schematic drawing of how the martensite is made to fit into the crystal by plastic deformation.
Three dislocations have moved all the way through the martensite. |
The (slightly distorted) cubes of the bcc lattice are not obvious because we
look at it from some special direction. Look at this picture
to see that you don't always see the cubes.
The black lines are meaningless. They just "guide the eye" to
recognize the dislocation (red line) and the shear deformation |
|
|
 |
The black arrows in the left drawing show what kind of atom movement by a shear deformation
will yield martensite. In addition, a dislocation in the sheared part (=pink martensite)
has already moved parts of the martensite one step to the left.
The drawing on the right shows how the bcc crystal
formed by shear (=pink martensite) is made to (almost) fit into the host crystal again by moving three dislocations through it on the dotted red planes. |
 |
The lower part of the schematic crystal is just "sheared" to the right
by moving all atoms on a horizontal plane by the same amount as indicate by the black arrows. The amount of shear increases
with the distance from the phase boundary that must form by definition.
A shear like this is related to what we called
"twinning" but we do not need to worry about
this here. All we should know is that shear as shown can be produced by moving a hell of a lot of special
dislocations (not shown) in a peculiar way. How that works is shown in the twinning link. |
|
 |
Why is the sheared off part now a bcc lattice? In the picture this cannot be seen.
Well,
for real crystals the shear is not just in the paper plane but has also some amount out of the plane. The whole thing is
just impossible to show edge-on. I
won't even attempt to draw it three-dimensionally in the proper perspective view; that would be far above my skills. It's
probably above anybody's skills since nobody so far has made such a drawing.
It's far easier to write down a few equations
that show it all and more. Provided, of course, that you can "see" what linear matrix algebra can "show".
Just believe me that with just shearing as shown schematically, you can make a
(very slightly distorted) bcc lattice out of a fcc lattice. That's what the right-hand part of the figure attempts to illustrate. |
 |
After a suitable shear, involving movement of a lot of atoms in military fashion,
the crystal still faces a severe stress problem. As the figure clearly shows, the sheared-off part that is now bcc martensite
would not fit at all into the still present fcc part surrounding it. |
|
 |
The right-hand side of the drawing shows how to deal with that problem. Run suitable regular
edge dislocations through it on the dotted
red planes, and you produce a structure as shown. Since those are regular dislocations, in contrast to the special
dislocations producing the original shear, you can run as many as you like through the martensite and it still remains bcc
martensite.
After enough dislocations did their work (and note that many of them will get stuck on the way!) the bcc
martensite part now fits into the "parent" fcc phase, if only with some effort. and some remaining stress. |
 |
Notice that I called the product of all this shearing and deforming "bcc
martensite" and not "bcc ferrite"!
|
|
 |
Why? Because after this whole process of shearing, involving moving a lot of
atoms at once in lockstep, i.e. military fashion, by running special dislocations through it, then running a hell of a lot
of regular dislocations through the newly formed bcc martensite, then squeezing everything elastically
(just changing the distance between atoms slightly) until the martensite fits into the space that its atoms formerly occupied,
we still do not have a nice bcc ferrite phase because
we also need to consider the carbon! |
|
 |
All the carbon that was present in the fcc austenite is still in the bcc martensite
because the shearing did do nothing to the carbon atoms. They stayed right where they were with respect to their iron atom
neighbors. After the martensite formation happened, they can't go away anymore either because it's too
cold. The net effect of that is that the bcc martensite is not really bcc, i.e. body-centered cubic
but a bit elongated in one direction. In other words, the carbon in the martensite is incorporated in such a way that it
induces a small normal deformation and instead of a cube we get a cuboid
as shown in the figure above
For being bcc, it is necessary to be strictly cubic (the "last "c" in bcc stands for cubic).
We do not have that anymore, we have a cuboid that the likes of me call a body centered "tetragonal" lattice. |
|
 |
What that means should be clear: In pure iron, or iron with very little carbon,
there can be no martensite. The martensite transformation discussed above would just be a messy and complicated way for
turning austenite into ferrite when there is no better way.
Martensite is only something special if there is carbon
involved. What that means is: |
| |
You cannot harden iron with too
little carbon since martensite
cannot be formed.
|
|
 |
Not all of the austenite can transform to martensite, by the way. After nucleation,
the rapidly growing martensite particles, often in the shape of plates, lathes
or needles, get into each others way and stop growing. So some austenite might be retained
in between, even so it is very unfavorable at room temperature. Also, if the quenching is not all that rapid, e.g. deeper
in the bulk, there might be enough time to form a little bit of ferrite / cementite in the usual way or even orderly pearlite.
What you get in the end is an awful mess of everything. And this mess is quite hard for reasons that should now be clear. |
|
 |
In an TEM electron microscope you can see it all. But even experts have problems
unraveling exactly what they see. Below are two pictures that serve only to show one possible general structure and the
inside of some martensite, |
| |
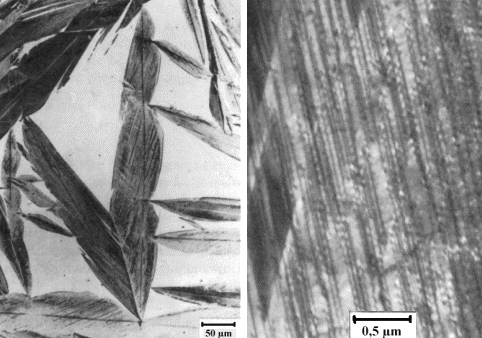 |
| What martensite looks like in the transmission electron microscope (TEM) |
| The black "feathers" are martensite as seen at low magnification in an electron microscope.
|
At high magnifications it becomes apparent that there is an extremely high density
of dislocations (the dark, somewhat wavy looking lines) inside the martensite platelets or lathes. |
| Source: "Werkstoffkunde Stahl"; p 155/157 |
|
 |
Describing martensitic transformations properly in mathematical terms, while not
too difficult for the initiated, does boggle the mind so much that we never attempt it in undergraduate courses. Thus nobody
notices that a lot of professors aren't too sure about it either. I will thus not go into this any more, except to summarize
by enumerating some important points: |
|
|
- In a general martensitic transformation some kind of crystal lattice
(bcc in our case) forms from some other kind (fcc in our case) by a military kind of
transformation. Martensitic transformations can be found in a lot of crystals besides iron. What exactly happens is rather
difficult to conceive by mere humans but a crystal has no problem in doing it. If a lattice type transformation is required
at some temperature by the phase diagram, but not possible by a diffusive transformation,
the only way left to approach nirvana is a "military" shear transformation, and that we call martensite formation.
If the driving forces are large enough, martensite formation will "just" happen. If that is not the case, the
high-temperature structure will become "frozen-in"
- While it is difficult to do the math, it is not impossible. Martensitic transformations are well understood by now and
there are many metals and alloys that produce their own kind of "martensite". For example, "shape memory
alloys" work by martensitic transformations. You may never
have heard about those things but they have saved the life of many people because "stents",
the little wire mesh things that keep your arteries open, are made from them.
- Martensite in steel is only extremely
hard because it is full of carbon and stuck dislocations. Martensite in very
low carbon iron is the ferrite phase formed by a martensitic transformation. Its properties are no different from ferrite
formed in the usual way by a diffusive transformation; it is rather soft. That is the reason why by quenching you can only
case-harden steel with a carbon concentration
not too low, inducing "true" martensite formation.
|
 |
So if you quench your whole steel sword blade, only
the outside and the thin parts—the edge—will cool down so fast that hard martensite is formed. The parts that
cool down fast but not fast enough for martensite, will also be somewhat harder because cementite or pearlite formation
in the normal way is still rather difficult. What one gets in this case is called bainite;
I'll get to that. Martensite only forms form the surface down to a certain depth known as quenching
or better hardness depth. This is easily measured with a so-called Jominy test, use the ink for details.
Now you have a hard (and rather
brittle) edge and you encased your whole blade in the hard stuff. That's why we call
this kind of hardening: "case hardening". |
|
 |
If you want to avoid having the whole surface of your blade turning very hard
(and brittle), you make sure that parts of it cool down more slowly - by coating it with some clay or whatever. Therewith
you provide thermal insulation of the coated parts and reduce the cooling rate to a
value where no or only very little martensite will form.
Here you have, in a nutshell, the Japanese way of making nihontos / katanas. |
 |
If your sword blade or whatever else you (case) hardened by quenching is now too hard and brittle, you temper it a bit.
We covered
tempering in some detail with the Al-Cu example. When you allow a bit of atomic
movement by tempering after quenching, the crystal moves its atoms around in such a way that it gets closer to nirvana.
For case-hardened quenched steel the first priority will be to get rid of the martensite by turning it into ferrite, and
that is done by casting out the carbon, forcing it to form cementite. Hardness then decreases but ductility increases. Stop
tempering at the right moment and you have the compromise between hardness and ductility that is best for you. |
|
 |
Here is a diagram that shows schematically what you can do
by quenching and martensite formation in comparison to slower cooling as a function of the carbon content. Of course, it
is only valid for the thin parts that could form martensite |
|
| .
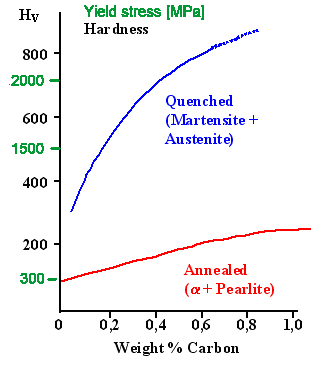 | | The two extremes for carbon steel. |
| Extreme hardness can be obtained by martensite formation (plus some retained austenite) upon
quenching. Cooling the mix slowly produces large-grained soft ferrite plus pearlite. Tempering after quenching produces
something in between. |
|
 |
With martensite hardening we go way
beyond of what could be done with all the other hardening mechanism. The stuff is harder than cementite, glass or granite! It is also perfectly brittle
like glass or granite; the first law of
economics is still enforced. |
|
 |
That explains why in the figure above the Vickers hardness was measured and not the yield stress. You can't measure
a yield stress if the material never yields but just fractures.
The yield stress numbers were just estimated from the
table I gave you long ago so you can compare (approximate)
numbers. |
 |
That leaves us with a little puzzle. Put a glass edge on a otherwise simple sword
and it is just as good as a true katana? Maybe—but how are you going to do that? |
|
 |
The great thing about hard but brittle martensite is that it is still nothing but iron and a little bit of carbon. We can make it directly from the steel we use, produce
it exactly where we want it, as hard as we want, and attached to the rest as well as could be. Try that with some steel and some glass!
|
| | |
|
|
Doing It |
 |
Understanding the theory of martensite formation is not an easy undertaking, even
so you can enjoy the comfort of your easy chair and your favorite beverage while trying to do so. Hardening a real piece
of steel by quenching it in your smithy is not an easy undertaking either, even if you quench your thirst with all the beer
necessary for that. Fortunately you, the ancient smith (and the modern one) can do that without the need to go through the
easy chair ordeal first.
You do not need to know anything about martensite and what happens inside your steel during
quenching. You just need to follow some working recipe. Smiths have quenched steel for the last 2000+ years without knowing
a thing about the theory behind it. They made babies the same way.
All smiths knew, however, that quench-hardening
is a tricky process that did not always work, for reasons unknown. Baby-making is not all that tricky in comparison but
doesn't always work either for unknown reasons until recently. This does not imply that there weren't all kinds of theories
around that sought to explain those deficiencies (not enough praying, praying to the wrong / Goddess, moon too full, evil
eye, witches, ...), it only implies that all those theories were utterly wrong.
What could go wrong with quenching?
Let's make a list:
- Your sword might come out straight and in one piece from the quenching bath but has not hardened.
- Your sword comes out nicely hardened in parts but not everywhere. Chances are that it is also bend.
- Your sword fractures and comes out in pieces that are possibly nicely hardened.
There is more that can go wrong. Maybe 95 % of your blade are perfect but there is an ugly hard blotch somewhere on
the blade where it shouldn't be. |
|
 |
This is rather upsetting because quench-hardening is done after
a lot of masterly work has already been invested in the blade. That all comes to naught if quench hardening fails since
you usually cannot repair what went wrong. It is therefore small wonder that a lot of mystery and magic developed around
quench hardening.
Since quenching did chance the properties of the steel a lot, and since it is hard to conceive that
the "nature" of the steel has changed a lot, it was reasonable if wrong to assume that the quenching fluid imparted
something crucial to the steel. It is thus small wonder that humankind came up with all kinds of (mostly disgusting) recipes
for quenching fluids. The link gives a taste treat. | |
|
| |
|
The Module in the link also shows that nobody ever quenched a sword blade by running it through a living body!
|
|
 |
In hindsight and armed with materials science insights is is easy to understand
why things could go wrong during quenching. Here is a list that elucidates what could cause "bad quenches" and
which factors play a major role:
- There is no hardening if there wasn't enough carbon in the outer parts that cool fast enough for martensite formation.
You will produce "martensite", yes, but it is not hard because it is simple ferrite.
- There is no hardening if your starting temperature was too low. Only austenite will transform to martensite and that
means that your blade must be above the transformation temperature of 727 oC (1341 oF) when it hits
the quenching fluid. And it must be at high temperature long enough to allow all carbon to dissolve.
- There is some hardening but not enough. You might have taken out the blade too early, performing a "slack quench
" (see below)
- There is no hardening if your steel contained a lot of carbon, like crucible
steel. Quench harden a wootz blade and what happens is that it will not get harder but is now perfectly brittle and
useless because the (primary) cementite is now lining the grain
boundaries.
- Local bending or warping happens if martensite forms inhomogeneously, i.e. only in parts of the blade or in different
amounts. That might be due to an inhomogeneous temperature distribution (e.g. parts of the blade were too cold) or inhomogeneous
carbon distribution. If you have other inhomogeneities, e.g. phosphorous-rich parts or non-uniform slag inclusions. local stress inhomogeneities during cooling could result that induce warpage.
- Any cooling produces stress in the sample; rapid cooling produces more stress than slow cooling. Fracture happens if
this thermal stress" exceeds a certain limit somewhere. Fracture
can be avoided if stresses are kept low or if plastic deformation occurs before fracture. Your blade will then be bent but
not broken. This may be the reason why a soft core is good for single-edged blades like katanas
- Straight double-edged blades with a symmetrical cross-section are easier to quench than single-edged blades since the
unavoidable stresses on the front and backside or on the two edges tend to cancel each other. That is not the case for a
katana, for example, where the thin edge cools far more rapidly than the thick back that is also thermally isolated by the
layer of clay applied to all but the edge region.
|
|
 |
There you have, in a nutshell, what you need for successful quenching. Note that
two independent factors come together:
- Martensite formation. Required is the right (medium) amount of carbon and
the right fast cooling rate. Other "dirt" like phosphorous or slag inclusions, while typically not good, can be tolerated up to a point.
- Thermal and structural stress.
Any phase transformation (like austenite Þ martensite) causes structural
stress, and any temperature difference (like between the outside and inside during quenching) causes thermal
stress. The two stresses add up. Stress causes first elastic deformation and then plastic deformation and fracture. Note
that thermal stress disappears completely as soon as the temperature is the same everywhere.
|
|
|
|
 |
Martensite formation and thermal stress are interlinked in a complex way. Martensite
formation is a phase change that causes stress, and it only happens when there is a large cooling rate that unavoidably
also produces large thermal stress. How the to stresses add up is not easy to guess, nor what will happen. In essence, however,
you might get one of the following four general cases:
- The combined stress causes essentially only elastic deformation. Then your symmetric blade emerges from the quenching
bath in the same shape it went in. There might still be stress in the martensite containing edge regions but they tend to
cancel each other. An asymmetric blade with martensite on one edge only might be somewhat curved.
- The combined stress causes some plastic deformation in some areas besides the always present elastic deformation. Your
blade then bends and stays bend even after the stress has gone or, as one says, has relaxed. But this bending is going the
right way and you now have an elegantly curved sword blade, e.g. a katana.
- As above, except that the plastic deformation is not going the "right" way, You now have a warped ugly blade.
- The combined stress exceeds the fractures stress somewhere and you end up with two or more parts. Not good.
Yes, quenching is complicated. If you want to dig a bit deeper, you need to consult the thermal
stress module. I tried to make it very clear and easy to follow. |
 |
I have said quite a lot about the art of quenching but never even mentioned the
quenching fluid, the magic and mysterious substance our ancestors considered to be of overreaching importance.
Well,
they were wrong. Very much so. The quenching fluid has only one function: to establish the cooling speed. It can do that
in three ways:
- Its temperature. Guess under what circumstances a hot potato will cool down faster:
If you throw it into hot or cold water? No more needs to be said.
- Of course, if you pitch a big hot potato into a little bit of cold water you are cheating. So you need to supply enough coolant. Pissing on your blade will not be enough.
- The same amount of liquid at the same temperature has one more distinguishing factor: its thermal
conductivity; how fast the liquid can transport heat from the hot to the cold site. That depends on what it is
and if it is vigorously agitated, streaming or just sitting there. The development of gas bubbles also has some influence
on quenching speed.
That's it. There is no transference of some "magical" stuff from your liquid to the steel, except perhaps
a marginal amount of carbon or nitrogen for a few nanometers below the surface. And you don't need that because you already
have plenty of carbon in your steel - otherwise quenching makes no sense in the first place.
However, various dirt
in your liquid might react with the surface of the blade to produce ugly spots (or to prevent this). So it really doesn't
matter from which river you took your water as long as it isn't too dirty. |
|
 |
It was the famous French scientist Réaumur
who figured that out and did away with 1000 years of disgusting superstition. Here
is an illustration to that.
Water is a pretty good quenching fluid and circulated water is more effective than still
water, of course. Circulated oil is more effective than still oil but less effective than still water. All the disgusting
stuff is in between.
More than that one does not need to know about quenching fluids. |
| |
|
© H. Föll (Iron, Steel and Swords script)