|
|
| |
What is Bainite? |
 |
What is bainite? In the backbone
I defined it as "in between halfway decent pearlite and fully developed martensite". That's about the best one
can do with just a few words. In German, actually, bainite is also known as "Zwischenstufengefüge" or "in-between-steps
structure".
Discussing bainite at any depth needs some tools that we don't have yet (like TTT
diagrams), so I will keep it simple here. |
|
 |
I have looked at a large number of books and papers to learn what, exactly, is bainite. The result is:
I'm not sure. Neither, it seems, is anybody else. Look at the table below and consider that something that needs that many
qualifiers cannot be all that well defined: |
| | |
|
|
|
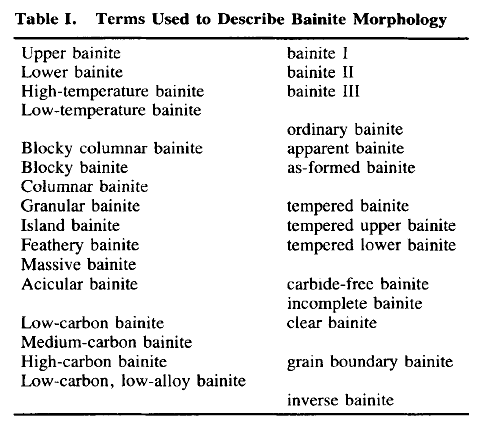 |
| Source: B.L. Bramfit and J.G. Speer: "A perspective on the morphology
of bainite", Metallurgical Transactions A, 21 (1990) p. 817 - 829 |
|
| |
| |
|
 |
Parts of the problem is that something called "bainite" is found in all kinds of
steel. It is actually more prominent in alloy steels
that contain all kinds of other elements in various concentrations than in simple carbon steels. And what you get in simple
carbon steels depends very much on the carbon concentration. |
 |
Let's go for the most simple steel imaginable in this context: plain carbon steel
at the eutectoid composition around 0.7
% carbon. No primary and secondary ferrite
and cementite, just a straight transition from austenite to ferrite and cementite. We also know what we will get if we cool
real slow or real fast:
- Slow cooling: Fully developed large pearlite grains as shown here.
- Very fast cooling: (in excess of 105
oC/s , to be precise): Fine grained martensite.
Somewhere in between we get bainite. |
|
 |
For starters that tells us that bainite must be a mixture of ferrite and cementite. The cementite,
however, is not neatly arranged in parallel plates but occurs in the form of many irregular precipitates inside small grains
of ferrite that are full of other defects like dislocations.
Continuing the figure about pearlite
evolution from the backbone, it will look very schematically like this: |
| | |
|
|
|
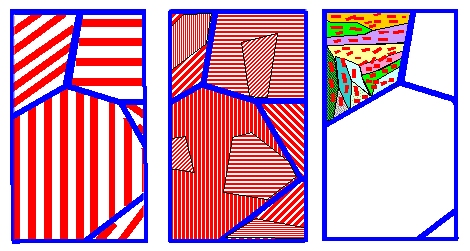 |
| Structure changes of of eutectoid steel with increasing cooling rate |
|
| |
| |
| |
 |
The blue lines show the grain boundaries in the austenite. As the cooling rate increases,
the pearlite structure gets finer and evolves into a fine grained structure with cementite precipitates shown in red (schematically!).
Structures like this we call bainite. Only one (former austenite) grain is shown in order not to overtax my artistic capabilities.
A lot of ferrite grains are nucleated within one austenite grain and start to grow simultaneously, often in preferred crystallographic
directions, leading to elongated and somehow interlocked grains (also known as "packets").
A little bit later, a lot of small cementite precipitates form, here and there, on grain boundaries and elsewhere, often
also with a preferred direction yielding longish or "acicular" shapes. The ferrite grains might be more like lathes
or more like plates, yielding somewhat different kinds of bainite known as "upper"
and "lower" bainite.
Schematically, we may envision structure formation to happen like this:
|
| |
|
|
|
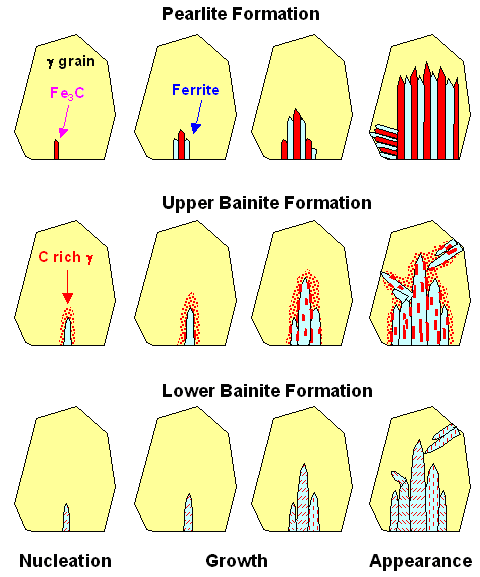 |
| Comparison of pearlite and bainite formation |
| Source: Similar pictures can be found, e.g. in "Metallurgy for Dummies". |
|
| |
|
| |
 |
The pearlite formation is clear.
As the cooling rate increases, the zebra structure gets finer and messier. Upper bainite can be envisioned as starting with
small ferrite nuclei, producing a carbon enriched zone in the neighboring austenite. The ferrite nuclei grow in certain
preferred crystallographic direction in the austenite matrix, producing elongated shapes. The supersaturated carbon precipitates
preferably at the (small-angle) grain
boundaries in the form of needles or "lathes". The process starts at many places and also in the interior
of the grain. The orientation of the longish ferrite grains is partially determined by the orientation of the original austenite
grain. For lower bainite, things are similar except that the cementite now precipitates inside the ferrite grains in the
shape of platelets. |
 |
What all that really looks like is shown below in a picture taken from Bain's
original work: |
| |
| |
| |
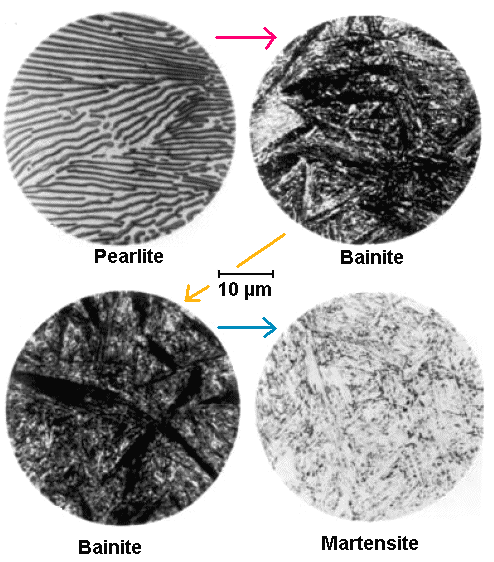 |
Microstructure development in eutectoid steel
with increasing cooling rate in arrow direction |
| Source: E.C. Bain: Functions of the alloying elements in steel, American Society for
Metals, Metals Park, Ohio, USA, 1939. |
|
| |
| |
|
 |
We have a simple eutectoid steel that is cooled down with ever increasing cooling speed along
the arrows. At the extremes we have nice "zebra" pearlite and martensite, in between
are two different kinds of bainite.
What I want you to note is that there is nothing to see for
you . This is only for experts (they see "dark etching
acicular aggregates"), and even then I wouldn't always trust their pronouncements. You also "see" that
with a light microscope you just don't see what is really going on. That's why a lot
of the names around bainite or other dense" structures" are a bit old-fashioned and obscure. A lot of guessing
was involved.
|
|
 |
If we now consider hypo or hypereutectoid steel, bainite-related stuff might already happen
with primary ferrite and so on. Take hypoeutectoid steel, for example, and you may end up with halfway decent ferrite grains
(formed at high temperatures) with a mess instead of nice pearlite in between. |
 |
So, maybe, we shouldn't focus so much on structural aspects but on other parameters
that allow to address bainite? OK - let's look at properties and products, and let's start with a quick look at the history of bainite. |
 |
It all began when in 1912 an international committee of noted steel experts tried
to define the various microstructures of steel and develop a suitable nomenclature. They came up with the by now familiar
terms: austenite, cementite, ferrite, pearlite and martensite, but also with things like osmondite,
ferronite, troostite and sorbite.
To quote the source given above: "A great deal of confusion persisted over the next
24 years", e.g. until 1936. Parts of the confusion simply resulted from the insufficient resolving power of light microscopes - you just couldn't see the details of all these structures.
One also didn't listen to the Germans. |
|
 |
So people simply invented names for typical structures they could see in their microscopes,
without knowing exactly what it was. That's where the "troostite" and "sorbite" come in, but also honest
if uninspired names like "dark etching acicular aggregates". The word "acicular" has come up before, but I always need to look it up, too.
It means "needle shaped".
Edgar C. Bain was pioneering
(together with Davenport) the nowadays extremely important TTT diagrams around
1930, when he was working in the research laboratories of the United States Steel Corporation in New Jersey. His coworkers
around this time started to call these "dark etching acicular aggregates"
(= longish things appearing dark in a microscope) "Bainite". That was smart. You still didn't know what those
darkish things were but you had a far easier time talking about it, not to mention that it didn't hurt your relation with
the boss. |
|
 |
In 1939, another famous steel guy, Robert Franklin Mehl, distinguished between "upper
bainite" and "lower
bainite", which he considered to form between (627 - 585) oC ((1161
- 1085) oF) and (400 - 200) oC ((752 - 392) oF), respectively. Upper bainite tends to have
more lath-like structures (i.e. ferrite grains), while lower bainite tends to platelet shapes. |
 |
If you think about that for a minute, you realize that Bainite is just a fine-scale
structure consisting of messily and quickly made fcc ferrite and some cementite that incorporates the surplus carbon that
could not be dissolved in the ferrite. If you cool down real fast, but not so fast that you get martensite, that's what
one must expect.
On second thoughts, one must also expect to get something similar for:
- Cooling down extremely fast to room temperature, producing martensite, followed by heating up or annealing the mess.
That allows the carbon to get out of the martensite (turning it into regular if messy ferrite) and form small cementite
precipitates all over the place. The result is what the Victorians in merry old England called "tempered steel"
- Cooling down extremely fast, but not to room temperature but to intermediate temperatures (like the one given above)
where martensite did not yet form but where it's already too cold for nice pearlite to develop. You still have austenite
then, from which you must expect that small and messy ferrite grains form, with small cementite precipitates all over the
place. This is known as "austempering
", short for austenite tempering.
|
|
 |
There are, in other words, many different ways to produce bainite. Of course, what you get
in the end following one or another bainite recipe is not exactly the same, it can be quite different in fact. But it always
tends to look like "dark etching acicular aggregates". The common denominator is that the re-arrangement of the
atoms is still mainly done by diffusion, albeit over short distances, and not by a "military" transformation as
for martensite.
Of course, not everybody agrees to that. My impression is that no statement about bainite is accepted
by all.
Just one random example. "Bainite is a phase (!!)
that exists in steel microstructures after certain heat treatments. It is one of the decomposition products that may form
when austenite (the face centered cubic crystal structure of iron) is cooled past a critical temperature of 723 °C.
Its microstructure is similar in appearance to tempered martensite. A fine non-lamellar structure, bainite commonly consists
of ferrite, carbide, and retained austenite. In these cases it is similar in constitution to pearlite, but with the ferrite
forming by a displacive mechanism (!!) similar to martensite formation,
usually followed by precipitation of carbides from the supersaturated ferrite or austenite."
There is indeed
an ongoing discussion if the ferrite grains or "packets" form by a diffusionless and very fast martensitic transformation,
out of which the carbon then diffuses, leaving ferrite behind, or gradually involving diffusion all along. I can't solve
this puzzle for you but it appears that the "diffusion" people have the upper hand at present. |
 |
We are only dealing with eutectoid carbon steel so far. What is going on in this
simple case is not all that clear. Bearing in mind that there is no such thing as plain carbon steel, we must now look at alloy steels. The issue doesn't get much
clearer, unfortunately, but it might get much easier to produce a bainitic structure. Restricting diffusion lengths by fast
cooling must and will leave a fine-scale structure and in a light optical microscope you still only see these dark blotches.
You also might retain a lot of austenite now, being metastable at room temperature but being there - and looking nice and
clean. Then you might top the confusion by referring to the structure as "austenite grains with inclusions of bainite". |
|
 |
Here is an example from a steel with about 1 % C, 1.5 % Si, 2 % Mn plus some Mo and Cr. They
were quenched to a temperature of, for example 200 oC (392 oF ) and then kept at this temperature
for a while. What you will get might look like this: |
| | |
|
|
|
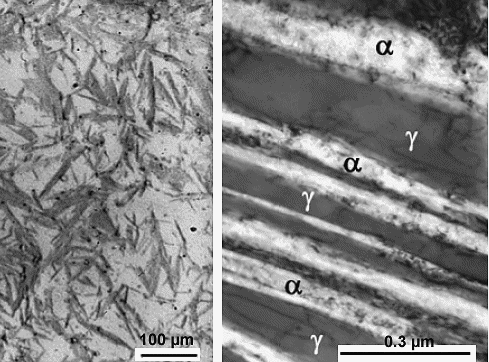 |
| "Bainite" without carbon precipitates |
| Source: F.G. Caballero, M.K. Miller, S.S. Babu. C. Garcia-Mateo: Atomic scale observations
of bainite transformation in a high carbon high silicon steel", Acta Materialia 55 (2007) 381–390
|
|
| |
| |
| |
 |
The dark stuff on the left are ferrite (= a) platelets in a retained
austenite (= g) matrix; on the right is a high-magnification view of a similar structure. There
are no cementite particles or other carbon precipitates because the carbon is still dissolved in the austenite.
You
may call the whole thing "bainite", you might call it "bainite in austenite", or whatever seems suitable,
using, perhaps, some of the qualifiers shown above. |
| |
| |
| |
What is Bainite
Good For? |
 |
A lot of "bainite" is produced for all kinds of applications. That's
not all that surprising, considering that we call a lot of microstructures in a lot of different steels "bainite".
What is a bit surprising, perhaps, is the observation that the large-scale production of bainite is mostly done by some
thermomechanical treatment ("hot rolling") followed by continuous cooling,
while most scientific studies employ isothermal transformation, i.e. keeping the steel
at constant temperature for some time (often quite long) after some initial rapid cooling to the desired temperature. It's
easier and neater for small samples but simply not practical for volume production. |
|
 |
What makes bainite interesting for specific applications? You should be able to make an educated
guess by now. The fine grained structure with lots of small precipitates is what you want if you strive for high hardness
(or yield strength, same thing) while still allowing some dislocation movement, preserving some ductility.
In other
words: You get hard steel that isn't completely brittle. It's not quite as hard as pure martensite, but then it isn't completely
brittle either. |
|
 |
It's more like the tempered
steel, in particular tempered martensite - and why not? Tempering martensite allows the carbon to get out of the martensite
or "unhappy ferrite", producing fine-grained messy ferrite and small cementite precipitates. You could call tempered
martensite just as well "bainite".
The big advantage of making bainite right away instead of first making
martensite and then tempering it, is clear: It's easier and cheaper! |
 |
So let's just say that a bainitic or "in between" structure of your
steel, whatever it is, might give you the optimal balance of strength, toughness and economy. |
|
 |
Those little nails you drive into the wall to hang pictures from, springs, high pressure vessels,
steam turbines, ...., and possibly swords are made from bainitic steel. Japanese katanas, having martensite edges can't
help but to have some bainite in between the martensite edge and the ferritic / pearlitic interior. |
 |
Just one last word to bainite usage. Besides the obvious parameters like yield
strength and ductility, there are some less obvious but just as important parameters like behavior at low temperatures (where all steels tend to become brittle) and behavior when
stressed for long times (called creep and fatigue). This will come up in the next chapter (the links will get you there
pronto), here we just note that bainitic steel might offer you some advantages with respect to these properties. |
| | |
|
© H. Föll (Iron, Steel and Swords script)




