|
9.5.2 Kinds of Cast Iron |
| |
Grey Cast Iron |
 |
The main alloying element besides carbon in cast iron is silicon (Si). It stabilizes
graphite and also improves some other properties (see below).
All the dirt that is contained in the primary "pig
iron" may still be there too, in particular our old acquaintances sulfur (S) and phosphorous (P). Some
manganese might have been added to take care of the sulfur in the usual way. Carbon is the important element and, as in steel
engineering, one sometimes defines a carbon equivalent concentration [Ceqi] by |
| |
| [Ceqi] | = [C] + | [Si] + [P]
3 |
|
|
|
 |
A high
cooling rate and a low carbon equivalent favors the formation of the white cast iron I have introduced in the preceding sub-chapter. It is the cheapest and simplest
variety of cast irons. Since it is very wear resistant, it finds applications in, e.g., slurry pumps, ball mills and grinding
mills or for the teeth in a backhoe's digging bucket. |
 |
More interesting, however, is grey cast iron. It tends to be formed for slow cooling rates and high carbon equivalents because
this allows to get closer to equilibrium and thus graphite is formed instead of cementite.
Graphite has no mechanical strength to speak of, and grey cast iron behaves mechanically like iron with voids or microcracks
instead of graphite. That causes cracks to go through the graphite inclusions. They are also deflected, and fracture surface
is rough and looks grey because a lot of graphite is exposed - hence the name. |
|
 |
The graphite forms already in the high temperature region, together with the austenite. Upon
cooling the austenite either decomposes into pearlite in the usual way, producing a ferrite - cementite mix, or, for very
slow cooling rates, into ferrite and graphite. In the latter case this secondary graphite just enlarges the primary graphite
inclusions already there.
Here is what pearlitic grey cast iron looks like: |
| |
 | | Microstructure of grey cast iron |
| Source: Internet article of Miguel Angel Yescas-Gonzalez and H. K. D. H. Bhadeshia; from the PhD
thesis work of Miguel Angel Yescas-Gonzalez. With friendly permission. |
|
|
 |
The dark longish objects are the graphite flakes. They typically precipitate in this kind
of rather stretched-out shape. In between one can just see the pearlitic structure of the matrix. If one considers the graphite
flakes to have no mechanical strength, they essentially are nothing but microcracks
that initiate fracture at the slightest "provocation". Grey cast iron thus is rather brittle, even so the
stuff between the flakes is good pearlitic steel. It is, however, less brittle (and hard) than white cast iron. |
 |
Grey cast iron is the most commonly used cast iron and the most widely used cast
material based on weight. Most variants have a chemical composition of 2.5 % to 4.0% carbon, 1 to 3% silicon, a little bit
of this and that, with the remainder being iron. More that 35 million tons are produced every year. It is clear why - consider
the list of properties: - Low cost.
- Good castability in general and in particular because the silicon increases "fluidity" when casting.
- Good hardness.
- Good wear resistance, to some extent because the graphite flakes act as lubricant. "Galling", the sticking
together of two metal parts "rubbed" very hard, does hardly occur.
- Good corrosion resistance in general and in particular if it contains silicon.
- Very good machinability since the bore chips or flakes
come off easily due to the graphite.
- Excellent damping capacity because the graphite absorbs energy, e.g. from vibrations.
- Less solidification shrinkage than other cast irons / steels because the graphite does not shrink much.
- Easy to weld.
- But: Low tensile strength and ductility and therefore not much impact and shock resistance.
|
|
 |
Obviously, we now must ask ourselves if we can do something about the last bad point? Having
progressed that far in this Hyperscript, you should be able to have some ideas in this respect. No??? Read on and learn.
Yes!!! Read on and see if your ideas would work. |
| | |
|
| |
Grey Cast Iron with Spheroidal Graphite. |
 |
The brittleness of regular grey cast iron results mostly from the sharp-cornered
microcracks supplied by the the (often interconnected) graphite flakes. If one could find a way to render the graphite spheroidal with no sharp corners, the cast iron should be far less brittle and even somewhat
ductile. How could one do that? There are two more or less obvious ways:
- Add something to the mix that hinders the graphite to form flakes.
- Anneal at sufficiently high temperatures to allow the graphite to assume the thermodynamically much better spherical
shape.
|
|
 |
If you go for the first point, it helps to figure out why graphite tends to precipitates
as flakes (or, if considered three-dimensionally, as "rosettes"). It might come as a surprise that this is a hotly
debated topic in polite scientific circles. Details thus are "complex" (meaning: not so clear even to experts)
but don't matter here. The question is: how can we prevent flaky graphite? The answer is: add minute quantities of magnesium
(Mg) or cerium (Ce) or .... (?) and the graphite inclusions grow more or less isotropically into spheres. Why? I don't know.
That's what it looks like: |
| |
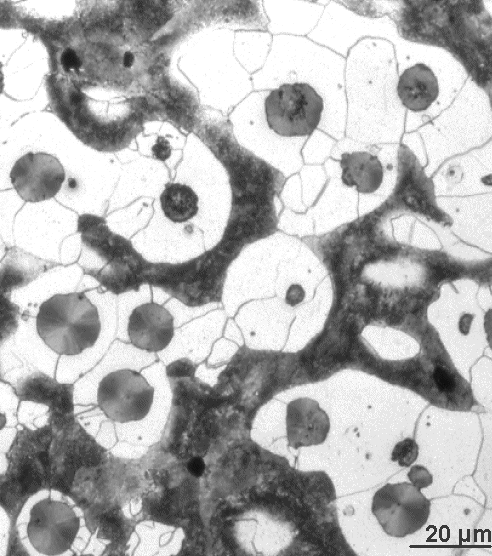 |
| Microstructure of spheroidal grey cast iron |
| Source: Internet article of Miguel Angel Yescas-Gonzalez and H. K. D. H. Bhadeshia; from the PhD
thesis work of Miguel Angel Yescas-Gonzalez. With friendly permission. |
|
|
 |
What we see are featureless round graphite particles, dark pearlite grains, and
whitish ferrite. The sample is an as-cast 3.5 % C, 2.5 % Si, 0,5 % Mn, 0.15 % Mo, 0.31 % Cu and 0.042
% Mg cast iron mix. The small amount of Mg is decisive for the spheroidal shapes of the graphite.
Note that
the graphite particles are always surrounded by a ferrite fringe. That simply happens because during cooling they "suck
out" the carbon that is contained in the pearlite / cementite that happens to be in their neighborhood, leaving back
the ferrite and a somewhat larger graphite particle. |
 |
Spheroidal graphite cast iron has much better mechanical properties than the normal
stuff. Its ductility increases at least five-fold if not twenty-fold compared to flaky-graphite cast iron, with all the
other good properties not much affected. In essence we now have a ferritic / pearlitic steel with spherical pores, and the
properties of the steel become important for the properties of the composite.
That should give you plenty of ideas - we know what we can do with steel,
after all. |
|
 |
For example, we might expect that annealing the stuff shown in the picture above
would allow the spheroidal graphite particles to suck more carbon out of the cementite that is still present in the remaining
pearlite. Ultimately only ferrite and graphite should be left. That works; what you get looks like this: |
| |
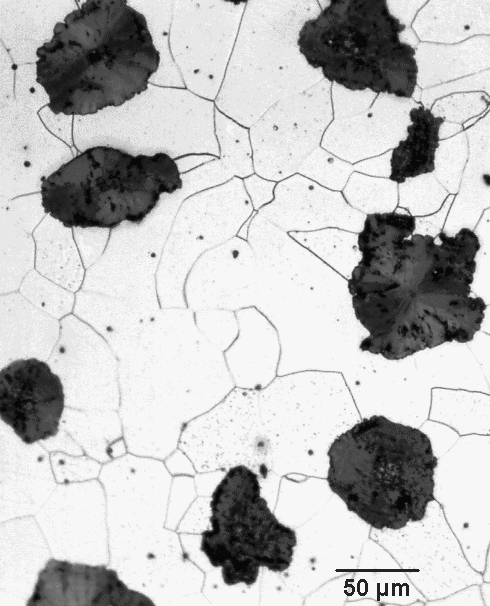 |
| Microstructure of annealed spheroidal grey cast iron |
| Source: Internet article of Miguel Angel Yescas-Gonzalez and H. K. D. H. Bhadeshia; from the PhD
thesis work of Miguel Angel Yescas-Gonzalez. With friendly permission. |
|
|
 |
It's the same sample as shown above; it just
was annealed some time (precise conditions unclear). No pearlite is left; we now have graphite particles embedded in (relatively
soft and very ductile) ferrite. You can clearly see that the originally rather spherical graphite particles have surrounded
themselves with somewhat irregularly shaped annexes - the carbon sucked out from the cementite. |
| |
This kind of ferritic spheroidal grey cast iron is no longer brittle but rather
ductile, as shown on the right. You can even twist it and forge a pattern-welded sword from it.
But would you want to
do that? Maybe not, because the material now is ductile but not hard. So use all the tricks you know for hardening steel,
For example, produce a hard bainitic matrix structure by employing
the "austempering" concept used so
successfully for regular (carbon) steel. Or try whatever else has worked for hardening steel. |
|
 | | Ductility of ferritic cast iron |
|
 |
You realize, of course, that I have just opened a huge treasure chest or a very
large can of worms, depending on how you look at the matter. There is no end to optimizing cast iron now. It is safe to
predict that we are going to see more and more advanced kinds with specific properties. |
|
|
 |
The catch, equally of course, is also obvious. It's no longer a simple and dirt-cheap
material. That's because the more sophisticated our alloying becomes, the more you must avoid useless or detrimental dirt.
And annealing always costs money anyway. |
 |
At the end, let's go back to the beginning.
How about making swords from cast iron?
Well, the need for good but affordable sword blades has sharply declined before
"high-tech" cast iron came into being and nobody has tried what one could do. I wouldn't be surprised., however,
if good blades could be made nowadays by using some optimized cast iron. Blades possibly better than what our ancestors
could make - but not as good as blades from modern steel. |
| | |
|
© H. Föll (Iron, Steel and Swords script)



