| |
7.2 How Do You Like Your Mix? |
|
7.2.1 Hypo and Hyper |
| |
Deducing Steel Structures |
 |
After we dealt with the eutectoid
composition , we must give a quick look to hypoeutectoid
and hypereutectoid steel during a slow
cooling down.
Let's start from a state point well inside the pure g phase in both cases. |
|
 |
- For the hypo ("below") case (blue state point), some primary
ferrite (light blue in the picture below) must form as soon as the g ® a + g
transition temperature is reached.
- For the hyper ("excessive") case (red state point), primary
cementite (pink in the picture below) must form as soon as the g ® g
+ Fe3C transition temperature is reached.
- You know by now that in both cases the nucleation of the new phase occurs most easily at the grain boundaries and especially
at the nodes of grain boundaries of the austenite. Later we will see that certain impurity
atoms also help to form cemenite.
|
|
 |
As soon as both steels hit the all-important 1000 K (727 oC, 1341
oF) transformation temperature, the still present austenite in both cases
must change to a ferrite-cementite mixture (striped in the picture below ). This is shown in the figure below. |
| |
 |
Formation of the structure of hypoeutectic and hypereutectic steel
during slow cooling down. |
|
 |
I'm going to discuss that figure in some detail by looking at the various state
points in the phase diagram and the (schematic!) structure going with them as shown to the left and right of the phase diagram |
|
 |
In both case we start with pure austenite or fcc iron with some dissolved carbon at a temperature
somewhat above 1200 0C (2192 0C) We can't image the structure
at such a high temperature but we can be sure that it consists of large grains without many defects. This is indicated by
the two topmost structure figures. |
|
 |
Some time after we started cooling, the state points hit the line separating the pure austenite
region from the two mixed phases regions a + g for the hypo, and g
+ Fe3C for the hyper composition. We now need to form some a ferrite or some Fe3C
cementite, respectively. How much of these new phases are needed will be given by the "lever rule" that we will
get to know quite soon.
Whatever, in the beginning we don't need all that much and the precipitation of the new phases
will start at good nucleation sites, in particular grain boundary nodes. This is shown in the second structure figure in going downwards.
|
|
 |
As the state points move to lower temperatures within the mixed phase regions, more ferrite
or cementite needs to be formed, and the new phases grow. This may happen along the grain boundaries (indicated in the third
structure figure on the right), more uniformly (right figure), or in some other way. |
|
 |
Eventually we hit the temperature where the transition to a ferrite
and cementite occurs in both cases (slightly below 1000 K in the figure). We know the composition of the g
phase at this point: it must have exactly the eutectoid composition
in both cases.
In the hypo case the carbon concentration in the austenite needed to increase
to get to this composition. Since carbon-poor ferrite was formed, this could happen. In the hyper case it is the same
thing, just with signs reversed. The carbon concentration in the austenite needed to go down, and it could do so because
carbon-rich cementite was formed.
We thus find just the proper amount of ferrite or cementite, distributed as shown,
before the transition occurs. |
|
 |
Now we go below the 1000 K transition temperature. In both cases the final structure must
consist of a ferrite and Fe3C cementite - just the amounts must be different.
The principle of supreme laziness dictates to leave
the primary ferrite or primary cementite as it
is. In both cases then only the remaining austenite with the eutectoid composition must
change into ferrite and cementite.
It will do that exactly as it did when we had the eutectoid composition from the
very beginning. In other words, it will change into pearlite. This is schematically
shown in the last structure figures above.
The pearlite then consists of secondary ferrite plus cementite, for the hypo case, and secondary
cementite plus ferrite in the hyper case. The primary ferrite and the primary cementite, respectively, remain unchanged |
 |
What we will get then is:
- For the hypo case (blue state point): pearlite embedded in primary ferrite
- For the hyper case (red state point): pearlite with some primary cementite in between.
Once more we assume that the new phases nucleate at grain boundaries and then grow (we will have a closer
look at that soon). What the structure really looks like is shown below.
First the hypo case: |
| |
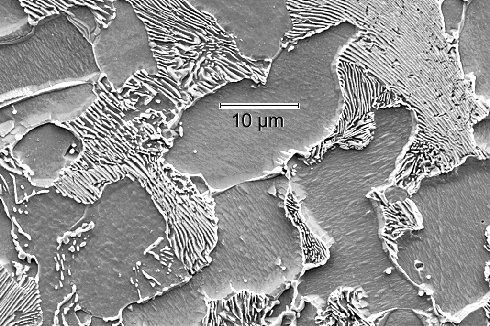 |
 | Structure of hypoeutectoid steel
|
|
| |
 |
The top picture was taken with a scanning electron microscope at a (medium) magnification
sufficient to resolve pearlite with a lamella spacing of about 0.5 µm. The bottom picture comes from a light microscope
at low magnification (200x).
The structureless grains are primary ferrite; the
"zebra" or black grains are pearlite, with an unresolved "zebra pattern" in the lower picture. Note
that not all grain boundaries are easily visible. |
 |
Now to the structure of hypereutectoid steel. Here is a light
microscope picture at medium magnification |
| |
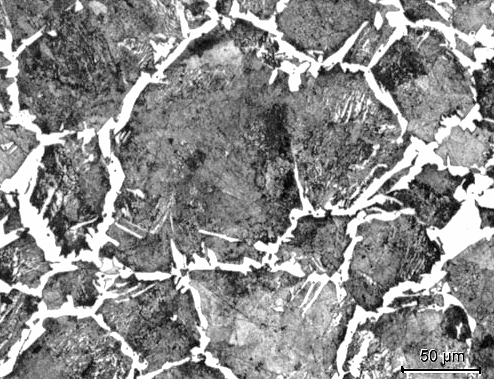 |
Structure of hypereutectoid steel (light microscope, medium magnification)
The
white areas a pure cementite |
| Source: Dr R F Cochrane, Department of Materials, University
of Leeds; by permission |
|
|
 |
The white stuff completely encasing the grain boundaries is primary
cementite. The rest (dark) is pearlite (not resolved) with a few pure cementite inclusions. |
| |
| |
| |
Pseudo Phases |
 |
My use of the word "pearlite" in all of the above cunningly prepared
you for an important new idea: |
|
|
For practical reasons, we
consider pearlite to be a
phase in its own right.
|
|
|
 |
Pearlite is not a real
phase, of course, since it consists of two
"real" phases - but what the heck. For all practical purposes pearlite behaves
just like a real phase. One might call it a pseudo phase but one usually doesn't.
|
|
 |
In the phase diagram, pearlite as a phase would be a vertical line, just like cementite. If
we draw pearlite as a phase into the the phase diagram below, the decomposition of the high temperature mixed phases into
ferrite and cementite (Fe3C) as shown several times before, can now be simplified into a decomposition into ferrite
and pearlite for hypoeutectic steels, or into pearlite and cementite (Fe3C) for hypereutectic steels, respectively.
Just to be on the safe side, here is the figure illustrating this |
| |
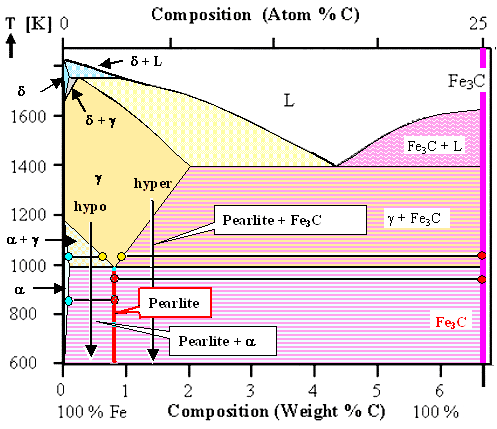 | Phase diagram for hypo- and hypereutectoid steel
with pearlite as additional "phase". |
|
 |
If you could follow me that far, you are no ready for parts of a basic Materials Science exam. Try it. It's fun. |
| |
| |
© H. Föll (Iron, Steel and Swords script)




