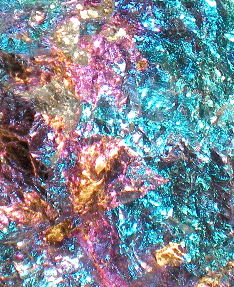| | Copper Ores |
 |
I won't even pretend to know a thing about copper ore deposits ands the geology
that goes with it. All I want to show is that an original upwelling of copper bearing stuff (typically some sulfides) gets
changed at the top part by exposure to groundwater and air. |
|
 |
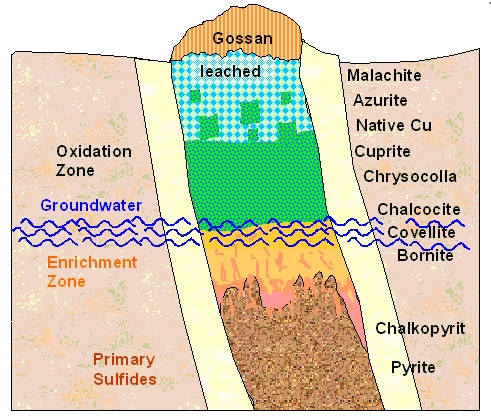
This extremely schematic picture gives an idea of what you might find at different
depths of a copper ore deposit. The general considerations here are also valid for other ores. |
| |
| |
 | | Distribution of Copper ores |
| Source: Pernicka / Weisgerber 2001 |
|
| | |
|
 |
Generally, the ores formed somewhere deep down tend to be sulfides.
They eventually were pressed up in cracks of the rocks ("hydrothermal veins") by geological events. Further up
the ore experiences atmospheric impact, and in this "oxidation zone"
these sulfides are exposed to the oxygen (and water) of the air, groundwater, and possibly large temperature changes.
In this oxidation or weathering zone, between the surface and the major ore body below, ores are oxidized to form various
rock minerals, including copper oxides, sulfates, carbonates (produced in the presence of limestone), and God knows what
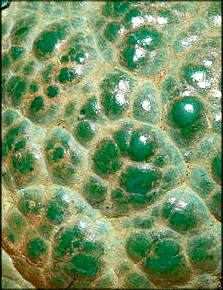
else. The best known copper carbonates are green malachite and the more unstable deep-blue azurite (not to be confused with
lapis lazuli). Copper silicates and turquoise – a rare, blue copper aluminium phosphate mineral, prized in itself
- may also be present. Native copper occurs at the surface of ore bodies and sometimes deeper in the ore body.
Some
of the compounds formed in the oxidation zone are water soluble. Rain leaches this stuff to below the groundwater level,
where it precipitates again, thus enriching the ore concentration at this level.
Deep in the ground where reducing
conditions prevail, copper sulfides like chalcopyrite, bornite and chalcocite dominate. These are now the major source of
copper.
The very top of the deposit gets completely weathered off, leaving only useless stuff behind. This region is
called "gossan" or "iron hat",
because quite often it is enriched with the stable iron hydroxide "limonite" (FeOOH) and therefore looks rusty.
A gossan was easy to recognize and gave a clear hint that digging there might be worthwhile |
|
 |
There is often no sharp boundary to the host rock. Hydrothermal action may decompose the ore-less
rock, and re-mineralization generates a corona, up to several 100 m wide, around the primary ore deposit that may also have
some metal-bearing compounds mixed in |
| |
| |
| |
 |
| Gossan in Australia |
| Source: company "Legacy Iron Ore" Limited. |
|
| | |
|
 |
Altogether more than 600 copper minerals are known. Here are some of the better
known ones. The most important one for today's mining is chalcopyrite. |
| | |
|
| |
| Name | Formula |
wt % Cu | | Malachite |
CuCO3•Cu(OH)2 | 57.3 |
| Azurite |
2CuCO3·Cu(OH)2 | 55.1 |
| Cuprite |
Cu2O | 88.8 |
| Chrysocolla |
CuO·SiO2·2H2O |
37.9 | | Chalcocite |
Cu2S | 79.8 |
| Covellite | CuS |
66.5 | | Bornite |
2Cu2S·CuS·FeS | 63.3 |
| Chalcopyrite | CuFeS2 |
34.5 |
| The better known copper minerals |
|
| | |
|
 |
Pretty much all of these minerals are colorful and if nicely crystallized rather
pretty: |
| | |
| |
|
| | |
|
 |
When you hike in the mountains you cannot fail to notice an exposed vein of copper
minerals. It's streaked green and blue with possibly some other colors mixed in. It is clear that ancient men must have
found that interesting. |
| |
| |
© H. Föll (Iron, Steel and Swords script)