Jominy Test and Hardness Depth
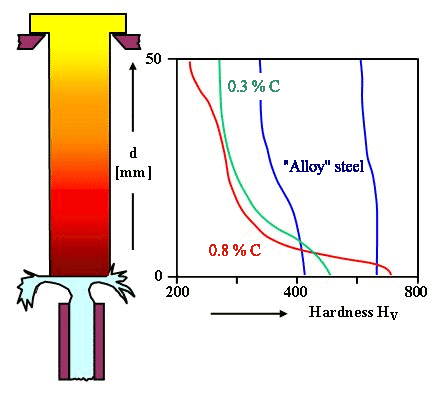 |
| Jominy test for hardening depth |
Now add some Cr, V, Mn, Ni, or Mo (or some other suitable elements), and if you do everything right, you may obtain the blue curves - steels with good hardenability and a much larger hardening depth.
The hardening depth is around 10 mm for the carbon iron (the exact number depends on how you define it) in this example. For the alloy steel it is larger, they are hard for at least 25 mm. However, the high-carbon steel is the hardest for the first few millimeters.
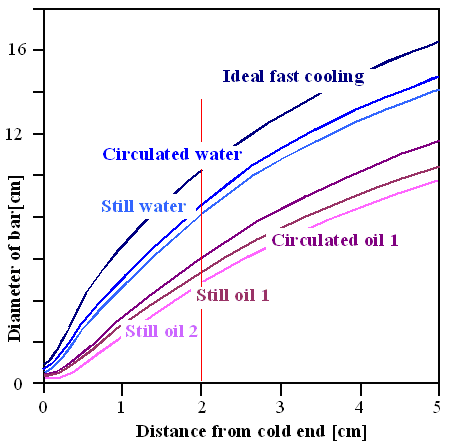 |
| Efficiency of coolants. See text for details. |
| Adopted from a "Key to Metals" article |
For example, if you can live with the hardness you get 2 cm from the end of a bar in a Jominy test (red line), your bar can gave a diameter of about 10 cm (blue line) and will have that hardness everywhere if you cool with maximal cooling rate ( liquid nitrogen, for example). Cooling in some well circulated oil is less efficient, the bar diameter goes down to about 6 cm (violet line).
We now could assess the efficiency of all that disgusting stuff the ancient smiths used for quenching their steel: various kinds of urine, blood, slaves..., keeping in mind that these kinds of coolant might also add a little carbon or nitrogen to the outer layer. You do the experiments.
I subscribe to the scientific insight, first understood by Réaumur around 1720, that the differences between different quenching agents comes only from their different thermal conductivity, i.e. how fast they can take the heat out of the steel.