| |
9.3.2 Maraging Steel |
 |
Using steel science, let's "design" high-tech steel once more to get a feeling for what can be done; we will stay with high-alloy steel. What we want
to make now is a steel that is: - easy to shape (meaning it is soft),
- extremely tough (meaning it is hard but still ductile),
- doesn't suffer from limited hardening depths.
- and can be welded.
That does sound like a big, fat contradiction in terms, doesn't it? |
|
 |
Well, yes, but there are ways. What we need is "obviously" a steel
that we harden after we have worked it into the desired shape. Since we usually work
steel around room temperature, the only option for a hardening process is now to heat
the steel to some temperature, and keep it there for some time. Temperature profiling once more, just the "wrong"
way around. So far we always softened a material by heating or tempering.
Or did we? Maybe you remember the example where an alloy got harder upon heating?
This yet-to-be-determined
process also takes care of the uniformity of hardening. While you can never cool down quickly and
uniformly, you can heat uniformly. Just heat up slowly. Since not much will happen before you reach the process temperature,
all is well. |
|
 |
What we are about to do is to make "maraging"
steel. The name is short for "martensitic ageing" and,
while fully correct, leads you straight into wrong associations. So far, "martensite" was code for "distorted ferrite containing carbon
and being extremely hard". While not wrong, it is a bit too special. I have pointed out before that martensite is only very hard if some carbon is stuck in there and distorted the crystal lattice. Undistorted martensite
in iron would just be ferrite and that is rather soft. You might think that the term "undistorted martensite"
is an oxymoron (a contradiction in terms) because it just means bcc ferrite. Well - you're wrong.
Nice clean bcc ferrite
that resulted from the nice clean fcc austenite preceding it on the temperature scale by a sudden martensitic
transformation is quite different from the bcc ferrite that is produced in the normal diffusion-driven way. It
is small-grained and full of twin boundaries and dislocations! Why? Because carbon or no carbon - all the problems with fitting sheared parts of the crystal into the space available
haven't changed.
What that means is that carbon-free martensite (= ferrite) is substantially harder then regular large-grained
ferrite with not too many dislocations. |
|
 |
We want our maraging steel to be relatively soft when its in the martensite structure.
That requires two things:
- No carbon. But there is no martensite (= ferrite) formation by shear in pure iron, so we need
- At least 19 % nickel (Ni).
Nickel, like manganese (Mn), cobalt (Co) and most of the nobler metals are known as g stabilizers, they produce phase diagrams known as open g-field systems (the link leads to the "alloy science
module"). What all that means is that with increasing alloy element concentration the transition temperature austenite
Þ ferrite (g Þ
a) comes down. That makes diffusive transitions more and more difficult and at some concentration
they just can't happen at all any more. That's when martensitic transformation takes over. Even that can be suppressed for
proper high-alloying and then leaves you with austenitic
steel.
The g Þ
a transformation for Fe - 18% Ni steels starts at 600 oC (1112 oF),
and that is just "too cold". These steels simply will not decompose peacefully into equilibrium austenite and
ferrite, even if held for very long periods at lower temperatures. Instead, during cooling the Fe-Ni austenite transforms
to hard but not extremely hard "martensite" with a bcc crystal structure. |
 |
That's already rather tricky but not doing you all that much good. The trick now
is to alloy optimized amounts of elements like molybdenum (Mo), cobalt (Co) or titanium (Ti) that are capable of forming
hard intermetallic compounds with nickel (Ni), things like Ni3Mo or Ni3Ti. Those alloy elements are
not doing much during the cooling down necessary to form the iron-nickel martensite but will provide for a lot of added
hardness when you now start the "ageing" bit, the annealing at the right temperature for the right time. It goes
without saying that before you start hardening by ageing, you first shape you relatively soft material into the form you want it to be.
|
|
 |
Of course, if you heat up to temperatures too high (roughly above 600 oC (1112
oF), you just form austenite again. But if you pick temperatures around 450 oC (842 oF),
you might form a lot of tiny intermetallic precipitates faster than your martensite decomposes into austenite.
If everything
is done just right, the precipitation
hardening mechanism, on top of the hardening already there by the martensite, will easily double or even treble the
hardness—without compromising ductility too much. This is what you see in the figure below: |
| |
| |
| |
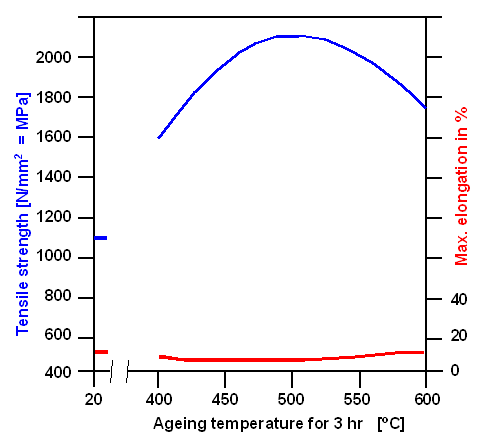 |
Yield strength (or hardness) development during
the ageing of maraging steel. |
| Source: From a data sheet of "Böhler". A Ni 18.5 %, Co 9%, Mo 5%, Ti
0.7% steel |
|
| |
| |
|
 |
A typical example of a good maraging steel is an iron alloy with 17 % - 19 % Ni, 7 % - 9 %
Co, 4.5 % - 5 % Mo and 0.6 % - 0.9 % Ti. Tempering or "ageing" takes place around 490 oC, producing
intermetallic precipitates. The term "ageing" was chosen in analogy to what we know from our old friend, the aluminum (Al) - copper (Cu) model system. |
 |
The initial development of maraging steels is attributed largely to work by C. G. Bieber, in the late 1950s. This work resulted in the first
two grades of maraging steel being introduced to the market. These steels contained 20 % and 25 % nickel, respectively,
with small additions of aluminum (Al) from "killing" the steel, and titanium (Ti) and niobium (Nb) for forming
precipitates. Since then many variants have been introduced. |
|
 |
Maraging steel, while used for all kinds of especially demanding applications, is still under
development and not all details are fully understood. In particular, adding substantial amounts of cobalt (Co) is rather helpful - see below - but what exactly cobalt does is not all that clear. Co may decrease
the solubility of Ni3Mo in the iron-nickel martensite but I'm not sure if that is the last word. |
| | |
|
|
|
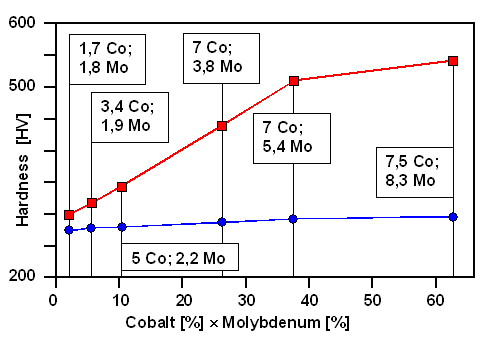 |
Combined action of cobalt and molybdenum on the
hardness achievable hy ageing..
|
| Source: Key
to metals |
|
| |
| |
|
 |
This is a rather curious diagram. It seems to indicate the hardening effect of proper "ageing",
i.e. of forming optimal densities and sizes of certain precipitates seems to increase linearly with the product
of the molybdenum and cobalt concentration. This means that it is proportional to each concentration by itself. This is
now one of those many little puzzles posed by experiments that theory needs to solve.
This link leads to a 1999 research paper, more
or less selected at random, that nicely illustrates some of the problems, including "banding",
something that will exercise us quite a lot as soon as I get to wootz swords; "dead heats" not amenable to "corrective
healing actions"; and "elimination of the research staff" in order to save money and make the problems go
away because nobody talks about them anymore. |
 |
Maraging steel with yield strengths of 1500 MPa or more are possible (corresponding
to a Vickers hardness up to 800 everywhere, not just in the "case"!) at a ductility of 6 % - 8 %!
|
|
 |
There is your material for your ultimate sword blade! Indeed, in modern fencing, three kinds of blades are used: the foil, the épée
and the sabre / saber. The all look quite similar to the layman and often are made from maraging steel; this might even
be required by international rules.
However, production, import, and export of maraging steel is also monitored by international
authorities because it is particularly suited for use in gas centrifuges for uranium enrichment. Lack of maraging steel
significantly hampers this process; ask your friendly Iranian politician. |
| | |
|
What Modern Steel Making Tells Us About
Sword Making in the Old Times |
 |
To summarize once more: Modern steels like the HSLA and maraging steels introduced
here resulted from scientific insights into structures and hardening mechanisms. They are many more steels like that; the
link will provide a lot of reading. Steel research and development is not yet finished but an ongoing and important enterprise.
The decisive factors for progress were the scientific revolution in the early 20th century and the insights into what crystals
are and how they deform around the middle of the 20th century.
In the 3000 plus years of iron and steel technology
before that, our forebears did not have the slightest chance to come up with something like micro-alloyed, stainless, or
maraging steels. |
|
 |
You just can't get far in designing and testing steels if you don't know what
happens, can't see your precipitates, and can't measure key properties in order to get quantitative data. In other words,
with no knowledge about deformation mechanisms, impeding dislocation movement by obstacles, and at best optical microscopes
instead of the whole bag of sophisticated micro-analytical tools we have today, you are simple "blind".
If
you want to make a better sword blade relative to the state-of-the-art, you can only
go by trial and error. This will only get your that far. So if your technique, based on centuries of experience gained by
your forebears, allows to make good blades most if not all the time, you eventually stop experimenting at all and cling
slavishly to the recipes that work. | |
|
| |
|
Japanese sword blades, for example, are just plainly amazing giving
the "blindness" of their forgers to what they were doing. The same thing, of course, is true for Celtic / Roman
/ Alemannic pattern welded swords and oriental wootz blades. |
 |
The down side of this is that no more progress will be made after your blade-making
process was declared perfect and canonized; e.g. in 16th century Japan. Is that bad? |
|
 |
Well, let's look at an example from a different but related sphere of human enterprise:
art. Painting in the West was pretty
good in the 16th century, just like sword making in Japan. Botticelli,
Cranach, Dürer,
Leonardo, Raphael,
Tizian, and many others come easily to mind (I did grant you a
halfway decent education)!
Some may claim that the masterpieces from these painters have never been surpassed but I beg to differ. Painting in the
West has evolved since then in a multitude of ways, and while I could do without certain works of art created since then,
I would not like to miss the French impressionists, parts of art noveau, and many other art styles quite different from
16th century painting. |
 |
In contrast, after apparent perfection was achieved, the art of steel as embodied
in blade forging did not evolve further in Japan - and that's a pity. In other parts of the world it did; you only have
to look the way the shape of swords changed and diversified. |
|
 |
Constant experimenting lead to progress in iron and steel technology, which in
turn eventually lead to guns, submarines, fighter planes, nuclear bombs, and so on.
You might consider that this does
not constitute much progress with respect to armed conflicts. If the good or the bad guys win is still a matter of chance
or taste; there are just a lot more dead innocent bystanders. I tend to agree.
But better steel also gave us cars, railways,
airplanes, boats, tractors, machinery of all kinds, radio and TV, computer tomography, painless drilling at the dentist,
minimal invasive surgery (I got a taste treat of that and liked it very much!), and so on. All of that would be impossible
without advanced steel.
And don't tell me that there is hardly any steel in a computer or cell-phone! The factory where
the microelectronic chips are made is full to the brim with machinery made from all kinds of steel! |
| |
| |
© H. Föll (Iron, Steel and Swords script)

