 |
The basic idea behind preferential etching
is to mark defects intersecting the surface by a small pit or groove, so they become visible in a microscope. |
|
 |
Start with a well polished surface that does not show any structures in a light microscope (including high magnifications and sensitive modes, e.g. phase or interference
contrast |
|
 |
Find an etching solution that dissolves your material much more quickly around defects than
in perfect regions (that is the tricky part). |
|
 |
Expose (= etch) your sample in this solution for an appropriate amount of time. What happens
will be something like this: |
| |
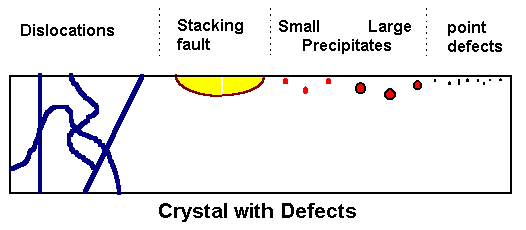
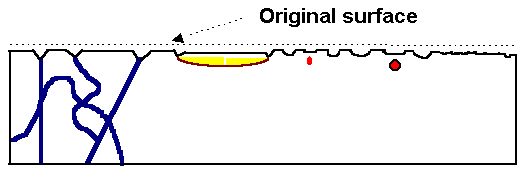 |
Model crystal with several kinds of defects intersecting the (polished) surface on top,
and surface
structure after preferential etching of defects. |
|
 |
After preferential etching you obtain well developed etch
pits (actually something looking more like pointed etch cones) at the intersection points of dislocations (including
partial dislocations) and the surface and etch grooves at the intersection line of grain
boundaries and stacking faults with the surface. Precipitates will be shown as shallow pits with varying size, depending
on the size of the precipitate and its location in the removed surface layer. Areas with high densities of very small precipitates
may just appear rough. Two-dimensional defects as grain boundaries and stacking faults may be delineated as grooves.
|
|
 |
There is a certain problem with grain boundaries, however: They may also
be delineated, i.e. rendered visible, with chemicals that do not preferentially etch
defects, but simply dissolve the material with a dissolution velocity that depends on the grain orientation (this is the
rule and not the exception for most chemicals). |
|
 |
In this case grain boundaries show up as steps and not
as grooves. Small steps and grooves, however, look very similar in a light microscope
and may easily be mixed up. |
 |
You may think: So what! - in any case I see the grain boundary. Well, almost right,
but not quite - there are problems: |
|
 |
Grain boundaries separating two grains with similar orientation with respect to the surface
would not be revealed. |
|
 |
The delineation of grain boundaries obtained under uncertain etching conditions suggests that
you delineated all defects - but in fact you did not. Delineation of grain boundaries
thus must not be taken as an indication that the etching procedure works and there are no defects, because you don't see
any! |
 |
Before we look at examples and case studies, two important points must be made: |
 |
1. Defect etching for many scientists is a paradigm for "black art" in science. There are good reasons for this view: |
|
 |
Nobody knows how to mix a preferential etching solution for some material from theoretical
concepts. Of course you must look for chemicals or mixtures of chemicals that react with your material, but not too strongly.
But after this bit of scientific advice you are on your own in trying to find a suitable preferential etch for your material. |
|
 |
Well-established preferential etching solutions usually have unknown and poorly understood
properties. They sometimes work only on specific crystallographic orientations; their detection limits for small precipitates
are usually unknown; they may also depend on other parameters like the doping level in semiconductors; and so on. |
 |
2. Defect etching in practice is more
art then science. |
|
 |
Beginners, even under close supervision by a master of the art, will invariably produce etched
samples with rich structures that have nothing to do with defects - they produced so-called etch artifacts. It takes some practice to produce reliable results. |
|
 |
But: Defect etching still is by far the most important and often most
sensitive technique for observing and detecting defects! |
 |
There are many routine procedures for delineating the defects structure of metals
by etching. Here we will focus on defects etching in Silicon; which is still the major technique for defect investigations
in Si technology. Some details and peculiarities of defect etching
in Si can be found in the link. In what follows we look at the power and possible mechanisms of preferential
etching in the context of examples from recent research. |
|
|
 |
Understanding the precise nature of swirl defects was deemed to be very important
for developing crystal growth techniques that could avoid these detrimental defects. |
|
 |
But etching alone can not give structural data, and other techniques as, e.g.,
transmission electron microscopy, could not be applied directly because the densities of swirl defects was too small (the
likelihood of having a defect in a typical TEM sample was practically zero). A combination of a special etching technique
and TEM, however, could give the desired results. |
|
 |
The power and the "black art" component of defect etching is nicely demonstrated
by the following development: A "special etch" which was simply the old solution, but cooled to about freezing
temperatures, did not produce etch pits (and thus remove the defect) for A-swirls, but hillocks (still containing the defect). |
| |
|
 |
The hillocks identified the precise location of the A-swirl defect. A special
preparation technique rendered the areas containing hillocks transparent for TEM investigations, and the structure
of A-swirls defects could be identified. They consisted of dislocation loop arrangements that were generated by the
agglomeration of interstitials. This gave the first direct evidence that self-interstitials are important in Si. |
|
 |
B-swirl defects could not be identified with this technique - their nature is still
not clear. |
|
 |
More about swirl defects and the
application of preferential etching can be found in an original paper (in German) in the link. |
| |
|
 |
The manufacture of integrated
circuits (IC)
involves many processes prone to introduce defects in the more or less perfect starting crystal. |
|
 |
All high temperature processes induce temperature gradients which lead to stress and thus
to a driving force for plastic deformation. Since the starting material is dislocation free, the decisive process is the
generation of the first dislocations which is much easier if small precipitates or dislocation lops are already present. |
|
 |
Thermal oxidation introduces Si interstitials with a strong tendency to agglomerate into stacking
fault loops, so-called oxidation induced
stacking faults (OSF). |
|
 |
All processes tend to induce trace amount of metals which will diffuse into the Si and eventually
precipitate. |
|
 |
Ion implantation destroys the lattice to a large degree up to complete amorphization. Even upon careful
annealing some defects may be left over. |
 |
As a general rule, all defects in the electronically active part of an IC
(roughly the the first 5 µm - 10 µm of the wafer) are deadly for the device. They have to be avoided and
that means that they have to be monitored first. The method of choice is preferential etching. |
 |
Lets look at an example |
|
 |
The pictures show a Si wafer with several defect types introduced during very early stages of processing.
Details are provided in the link. |
| |
|
 |
A few more example are provided in the links. They might be a bit unconvincing,
but be aware that looking into an actual microscope gives you much more information than what can be captured in a few pictures. |
|
 |
Development of stacking faults in bipolar transistors |
|
 |
Precipitates and other defects |
 |
We are now able to compare weaknesses and strength of preferential
etching for defect detection: |
| |
| Strength |
Weaknesses |
- Simple and cheap
- Rather sensitive
- Applicable to large areas
- Needs no special knowledge (as e.g. TEM)
| - Black art
- Detection limit unclear
- What you see must be interpreted
- Problems with artifacts
- Mechanism not clear
- No systematic developments of etches
|
|
 |
One last example serves to illustrate the "what
you see must be interpreted" point. Shown is a complex defect composed of stacking faults, dislocations
and possibly a microtwin in full splendor in a TEM micrograph
(left), and a schematic outline of what the preferential etching would look like in an optical microscope. |
| |
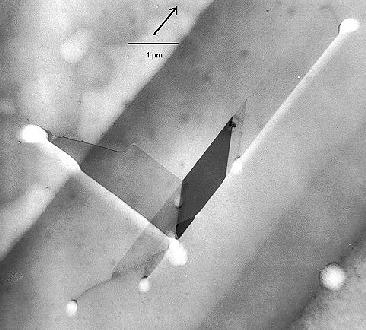 |
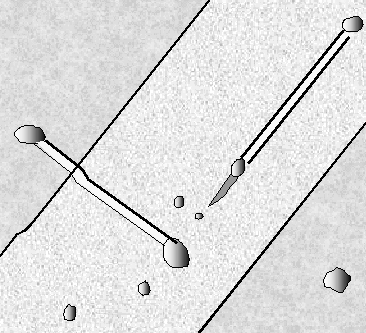 | | TEM micrograph |
What you would see with preferential etching
Since the etch pits are smaller than 1 µm,
they only would appear as blurred black-white structures |
|
|
 |
The planar defects are inclined in a thin foil; what one sees is the projection. One surface was preferentially
etched; at the intersection of the defect with this surface the etch features can be seen as bright areas (the sample thickness
is smaller at etched parts). The stacking fault lines will be clearly visible in an etch picture, but the various dislocations
involved are etched with different strengths. |
|
 |
It will not be possible to conclude from the etch pattern alone on the complexity of the actual defect.
This stacking fault assembly corresponds to some extent to the etch pattern shown in the development of stacking faults
in bipolar patterns given in the link. |
 |
Chemical etching on occasion is driven to extremes - simply because there is no
alternative. The link leads to an advanced module, where a particular tricky
case study is presented |
|
|
 |
Chemical etching, as any chemical dissolution process, is an oxidation-reduction
process expressed in chemical terms. Carriers are transferred from the substrate to the chemicals, new compounds form and
go into solution. The paradigmatical model for these processes is anodic dissolution
under applied bias, where the carriers are supplied by a controlled external power source. Maybe a way towards the understanding
of preferential etching comes from the electrochemistry of the specimen? |
 |
Anodic etching has been studied to some extent in Silicon. It leads to a rather
unexpected wealth of effects that are at the focus of some current resarch projects. The experiment is simple: |
|
 |
Bias the (p-type) Si sample positively in some electrolyte that contains hydrofluoric
acid (HF). The HF itself is "contacted" by some inert electrode, e.g. a Pt wire, which establishes
a closed circuit. |
|
 |
The Si-HF- junction behaves to some extent like a Schottky
junction; current flow, however, is always accompanied by a chemical reaction. The current density first increases steeply
with the applied bias, then reaches a maximum (called jPSL; PSL stands for "porous
Si layer") and decreases again (that is when the analogy with a Schottky junction fails), goes through a second
maximum (called jox) and finally starts to oscillate . |
|
 |
In the "forward" regime of the junction, the reaction is the dissolution of Si (in reverse
condition it is H2 evolution). |
 |
If a polished specimen that was subjected to a current density considerably smaller
than the first peak value is inspected after some etching time, its defect will be revealed in a way reminiscent of purely
chemical etching. This can be understood (in parts) by considering current flow in terms of diffusion
current and generation currents as introduced in basic pn- (or Schottky)-junction theory. The major ingredients
for anodic etching are shown below. |
|
|
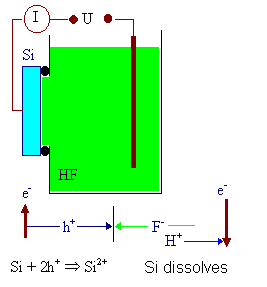 |
 | Basic experimental set-up, current flow
and chemical reaction |
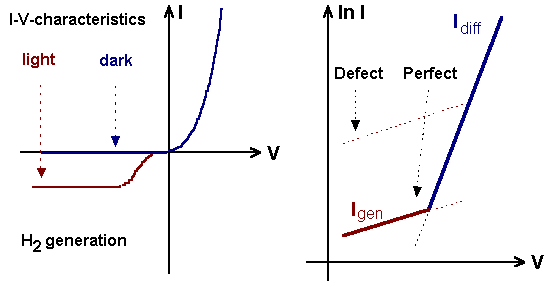
Measured I-V-characteristic and theoretical plot of ln I vs.V with diffusion
and generation currents. Around a defect the generation current is larger than in perfect Si. |
|
 |
Preferential defect etching thus can be understood in terms of current flow: At
small current densities the generation currents are larger than the diffusion current, the area around electronically active defects (i.e. defects that generate carriers) should be etched more deeply
and etch pits should appear. At larger current densities the differential etch rate should disappear. The experiments support
this view to some extent; the link contains some results |
|
 |
General results of anodic etching |
 |
The consideration of the influence of defects on a Schottky junction suggests
a different approach to the detection of electronically active defects: Measure the local leakage current or radiation induced
current of a junction. This can be done by injecting current locally by an electron beam through a thin Schottky barrier
while measuring the induced current. Electronically active defects will recombine more carriers than the defect-free regions,
the current will be locally reduced. |
|
 |
This method exists and is called "electron beam induced
current" technique (EBIC) if a scanning electron microscope is used as the basic instrument. If a scanned light
beam is used, we have the "light beam induced current" technique
or LBIC;
the mainstay of solar cell development with poly crystalline Si. |
|
 |
The principle of EBIC is shown in the link. |
|
 |
If one compares anodic etching, chemical etching and EBIC, much can be learned about defects and
the detection methods, but many questions remain open. Some examples
are given in the link |
 |
Anodic etching is still a virulent research issue within the context of the general electrochemistry of semiconductors. |
© H. Föll (Defects - Script)

