|
Science of Welding Steel |
| |
The General Problems |
 |
In this module I look cursorily (very!) on the art and science of liquid
or fusion welding of steel. It has to be superficial and sketchy because there is no
other way to treat this monstrously huge and complex topic in just a few pages. Moreover, I have never in my life welded
anything myself. |
|
 |
I have looked at welding before in this Hyperscript:
- Here you find some general remarks about
welding as one of the major techniques to join two pieces of metal. If you are completely unfamiliar with welding, read
that first.
- This module looks at weldability
as one of the major properties of steel.
|
 |
There are many ways of fusion welding. Here I restrict myself to "electrical"
or arc welding. Essentially you draw a powerful electric discharge, an arc,
between a welding electrode and the metals to be welded. When we weld, we liquefy surface-near regions of the two pieces
of the metal to be welded and parts of the electrode we use for arc welding. Generally we try to melt as little as possible
of the materials to be welded, having mostly liquid electrode material in the resulting mix. The liquid flows into the region
to be joined and solidifies with a cooling rate that tends to be rather large. Why? First, the melted volume is not so large
so it has a large surface to volume ratio. Second, most of the surface / interface is in contact with a good heat conductor
- the steel to be welded.
In a first approximation you must expect that the microstructure of the solidified weld seam
will be similar to that obtained by casting the composition, followed by rapid cooling.
And now you have the first problem with welding: |
| |
| |
| |
|
The microstructure of the weld seam tends to be quite different to what you have in the bulk.
|
|
| |
| |
|
 |
That is not necessarily bad. If your weld seam has a higher yield strength but smaller ductility
than the bulk material, it won't matter much if there is no need for further deformation. However, if major stress builds
up in the product later, like in a car accident or here,
the steel will now come apart at the weld seams. Of course, the unavoidable thermal
stresses encountered during cooling must not exceed some critical limits in your weld seam or cracking might occur right
away,.
If your weld seam has a lower yield strength than the bulk material .... you can figure that out yourself. |
|
 |
What compounds the problem is that solidification may start from both
solid sides, with the center of the weld line solidifying last. Since the solid-liquid interface sweeps impurity atoms along
because of segregation effects, the center of the weld is enriched
with impurities that have a large effective segregation coefficient, and thus is possibly rather dirty - and then weak! |
|
 |
You can counter the difference in microstructure to some extent by choosing an electrode with
optimal compositions (not necessarily fully identical to that of the steel to be welded) and countless tricks I can't go
into (mostly because I don't know them). |
 |
The difference in the microstructure between the bulk material and the solidified
materials is just the first problem. The second
problem stems from the different microstructure in the Heat Affected Zone or HAZ. That is the area
next to the formerly liquid parts that did not liquefy but still got rather hot. If
you think that the HAZ cannot possibly be a bigger problem than the microstructure of the liquid part, think again. |
 |
The third problem results because (liquid)
welding invariably produces major thermal gradients. In other words: the temperature changes enormously over small distances.
Thermal gradients invariably cause stress until they disappear,
and stress causes problems up to and including fracture. |
 |
Those are the obvious problems. The not-so-obvious
ones result from the fact that you might get all kinds of unwanted stuff into the molten part: Oxygen and nitrogen from
the air, stuff from the flux you use to prevent that, and in particular hydrogen from the welding process itself (see below).
Since the liquefied part is vigorously stirred by all kinds of processes - you have a tremendous power density, after all
- whisking in gases is easy and always detrimental. |
|
 |
There is little choice: you must protect the liquefied part from direct contact with the air.
This is where all those "tricks" come in, and where welding turns into an art. In essence you blow non-reactive
gases over the liquefied part or even a liquid flux that hopefully does not do anything bad to the steel. I'll give examples
of that right below.
It is not surprising that welding wasn't used right away when the age of steel started around
1850. It had to wait until 1920 or so before it was developed to a level where it could push steel technology to unprecedented
heights. So, before I go briefly through the various problems encountered in welding, let's get one thing right: |
| |
| |
| |
Welding is a major enabling technology! Without welding we would not own cars, for example.
|
|
| |
| |
| |
A Few Words to Welding Hardware |
 |
Welding for quite some time was mainly "flame"
torch welding". Acetylene (C2H5) and oxygen from two high
pressure cylinders are fed to a torch or blow pipe and ignited. The temperature in the flame is hot enough to melt iron
and steel and thus allowed cutting and / or welding - provided you knew what you were doing and didn't set yourself and
everything else on fire. |
|
 |
Torches are still used for cutting but rarely for welding anymore. That is now done by a plethora
of methods, most of which use electricity in one form or other to supply the energy density
necessary. Here I will only look very superficially on arc welding. |
 |
In essence, you draw an electrical discharge - an arc - between the material to
be welded and a welding electrode. The arc is confined to a region scaling with the diameter of the electrode and thus might
cover an area of some 10 mm2. The power delivered is voltage times current. With voltages around (20 - 30) V
and currents of a few 100 A, you have something like (2 - 10) kW/cm2 or more. Your electrical range, for comparison,
gets quite hot with just about 1/1000 of this kind of power density.
So your electrode
melts, and so does that part of the "parent" metal that is struck by the arc. Have your electrode made from the
same material as the steel you want to weld, and you are in business, it seems. |
|
 |
Not really. You can weld your steel, yes, but chances are that you are not going to be happy
with the quality of the weld. For starters you should protect the surface of the liquid steel from contact with the air,
as already mentioned above. The first measure for doing this is to use a "covered"
electrode. |
| |
| |
| |
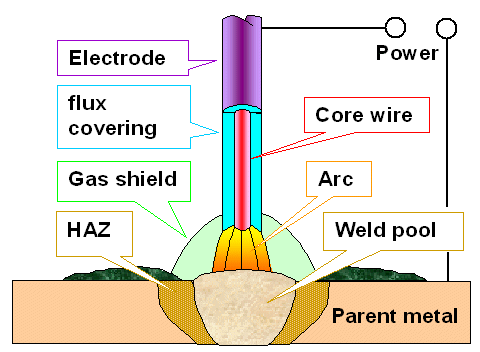 |
| Arc welding with a covered electrode (HAZ = Heat Affected Zone) |
|
| |
| |
 |
Early welders found out that covering your steel wire electrode
with paper, mud, concoctions from tobacco stalks, and so on, might produce better welds. Not always, of course, but often
enough to indicate that a proper covering could be beneficial.
Nowadays we use three main kinds of coverings, mostly
mixed with some powdered parent metal, too:
- Cellulose (instead of paper). It decomposes in the arc, liberating hydrogen and
other stuff. Little slag or flux is produced, and the hydrogen gas shield protects the molten metal. It also provides for
some other benefits but causes major problems, too (see below), especially in high-strength steel. It is a good electrode
for welding mild steel.
- Rutile is the major form of naturally occurring titanium dioxide (TiO2).
It works nicely for not too complicated welds and materials by producing protective flux / slag. However, it also produces
some hydrogen and that might be bad.
- Basic (as opposed to acidic) stuff like calcium carbonates or fluorides. Carbon
dioxide gas (CO2) plus plenty of flux (turning into slag) produced in the arc shields the liquid metal. The hydrogen
evolution can be low. Not an easy process for various reasons but the electrode of choice for welding high-strength steel
and complex shapes.
|
|
 |
What becomes clear is that welding is not as easy as it looks. If you consider to do some
welding, it should be apparent that there are a lot of parameters that you must get just
right. If you go for arc melting, you must pick the right electrode, the proper voltage and current, the right geometry,
the right speed for moving the electrode along (always keeping the distance right), the proper pre-treatment of the parent
metal (including, maybe, some pre-heating to temperatures above room temperature), and so on. If you got everything right,
you might get a good-looking weld seam. |
 |
Now I can get to the crux of the welding problem: Even if you succeeded to produce
a composition and microstructure of the weld seam (plus heat-affected zone) that is not too different from that of the parent
metal, you still might be in in for major trouble: Your weld seam may develop cracks! |
|
 |
It goes without saying that this is deadly. If your weld is cracking while you are still at
it, you can just forget about it. That is pretty bad but things can be even worse: your weld cracks after
you finished it. Just about when the weld finally reaches ambient temperature or - horror - a few days
after the act!
There are several reasons for weld cracking, many related to hydrogen.
And now we get to the more involved science, art and mysteries of welding. What, exactly, causes these cracks - and how
can I avoid them? |
| |
| |
| |
Welding and Crack Development |
 |
If cracks occur in your weld seam or in the heat affected zone next to it, you
know that you encountered fracture in a nominally ductile material. I have given you three fracture modules already, let's
quickly recount the important points:
- You need to have a critical stress level scrit to allow a crack
tip to propagate. This critical stress must be lower than the stress syield
needed to move dislocations. The infamous ductile-to-brittle transition bears witness to this.
- "Crack tips" can be just that - the tips of "real" cracks: More important, they can be the "tips"
of tiny defects that we called "nanocracks" and that
initiate or nucleate crack formation.
- The critical stress gets smaller for smaller surface energies
and for larger pre-existing nanocracks. In a more advanced theory all these parameters are lumped together into
a "stress intensity factor".
So why should a weld seam crack? There are two major reasons:
- For all the reasons that I already have covered somewhere. All metals can develop cracks, after all, under certain conditions.
- Because hydrogen (H) got into the material and causes all kinds of problems that
finally produce cracks.
I'm going to treat "normal" cracking very briefly and then go a little bit deeper into hydrogen-induced
cracking. |
 |
"Normal" cracking can occur without the "help" of hydrogen.
It often reveals itself as "center-line" or solidification cracking, and happens more or less shortly after solidification. This is what it might
look like: |
|
| |
|
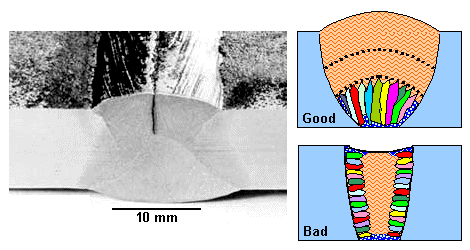 |
| Center-line cracking and extreme solidification geometries. |
| Source: Thanks go to TWI in Cambridge, England, for sharing the picture. |
|
| |
|
|
 |
There are several reasons for this kind of cracking to occur. First of all, there are always
high stresses from the thermal gradients. After solidification the hot parts shrink and then are under tensile stress. If
the center of the weld seam solidifies last as in the lower of the two schematic drawings, segregation
will have enriched it with all kinds of impurities, and that typically makes a material more brittle. Moreover, if the crystallites
grow from the outside to the center, the two crystallization fronts meet in the middle, possibly causing a weak and porous structure there for the reasons given in the link.
All of the
above is tied to the geometry of your weld seam (or bead, as the experts call it). This is schematically illustrated above
for two extreme solidification geometries.
Adjusting conditions (voltage power, geometry, ...) to "just right" values will allow to avoid the problem, particularly in mild
steels.
There are some other "normal" cracks. They are mostly related to "bad" welding that produces,
for example, slag inclusions or largish manganese sulfide precipitates, and I won't go into that. |
 |
Let's now look at the real problem: hydrogen-induced cracking. It comes
under many names, e.g. hydrogen assisted cracking, heat affected
zone cracking, cold cracking or delayed cracking.
Extremely simplified, what happens is that cracks develop in the heat affected zone
(often close to the old solid-liquid interface) when it's "cold", i.e. the temperature is below
about (150 –100) °C ((200 - 300) °F). The susceptibility to hydrogen-induced cracking actually maximizes
at about room temperature, and it may even take a day or two before these cracks develop.
Here is what it might look like: |
| |
| |
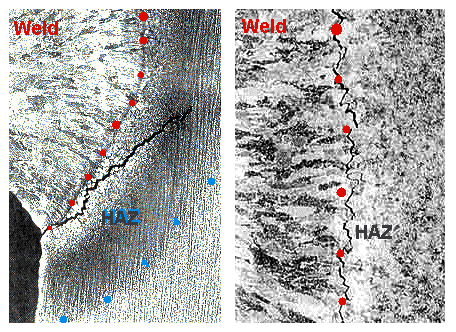 |
| Center-line cracking. Red dots mark the interface Liquid - HAZ. |
| Source: Internet at large |
|
| |
| |
|
 |
All this hydrogen related cracking is really bad, in particular because it get's worse for
more advanced (high-strength) steels. Innumerous studies have been made and are being made since an outrageously large amount
of money is tied to that. Consider that only one crack of this kind appears in the many thousands of welds in a 1000 mile
long pipeline that is submerged in the Baltic. Repairing the pipeline will require serious money. |
 |
That's why the welding community came up with all kinds of empirical equations
and graphs that relate weldability to composition. One example is shown below: |
| |
| |
| |
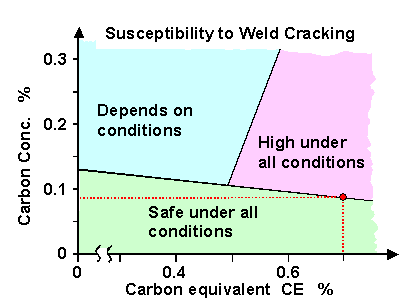 |
| CE | = [C] + | [Mn] + [Si]
6 | +
| [Ni] + [Cu]
15 | + |
[Cr] + [Mo] + [V]
5 |
|
[..] denote wt% concentrations |
|
| |
| |
|
 |
The figure is self-explaining and demonstrates nicely that you run into serious welding problems
with increasing carbon and carbon-equivalent concentration. It shows, for example,
that plain steel with more than about 0.6 % carbon cannot be welded. Here is one of the reasons why low-alloy steel is so
popular.
The Graville diagram is, of course, just a guide and not always "true". Many more rules similar
to the one embodied in this diagram exist - but I won't go into this since I do not intend to write another hyperscript,
considerably longer than this one, about hydrogen and welding. |
 |
Instead I will try to answer all your questions that must have come up by now:
- Why should there be hydrogen around during welding?
- Why should hydrogen, if it is around, do all these bad things?
- How does hydrogen-induced cracking work?
|
 |
The first question is the easiest one to answer.
At the extreme temperatures and power density of the arc, hydrogen is produced from about anything that contains it. For
example from organic stuff, like the cellulose that might cover the electrode, and in particular from water. Some water
is always around; the water vapor contained in the air will already be enough to cause trouble. Even if you use electrodes
covered with nominally hydrogen-free stuff (e.g. the basic electrode), it will have absorbed
some water from the air. The surface of your steel also has a water vapor layer on it and possibly hydrogen containing oil,
grease, dirt or paint, not to mention hydro-oxides called rust. You must bake your electrode for a while to drive out the
water and clean and even pre-heat your steel and so on, if you want to minimize the amount of hydrogen produced during welding. |
 |
The second question also has an easy answer
for starters. Hydrogen does bad things because:
- It is highly mobile. At high temperatures above, let's say, 500 oC (932 oF), it covers distances
of almost mm in the first second. At room temperature it still covers some 10 µm in the first second. The graph below
is an extended version of an old
figure and shows that the diffusivity of hydrogen is way above everything else.
- Hydrogen dissolves easily in the melt and has a high solubility in the solids at high temperatures. However, its solubility
drops dramatically with decreasing temperature, and it is higher in austenite than in ferrite.
|
| |
|
| | |
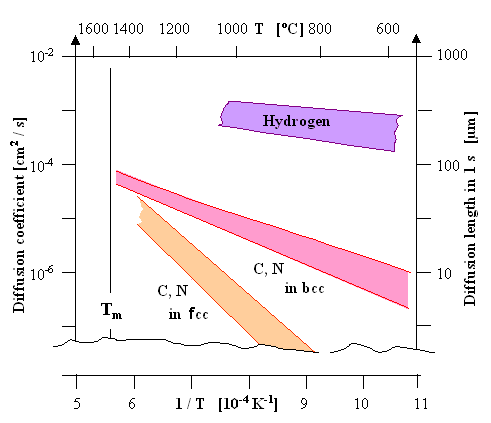 | | Hydrogene diffusivity at high tempertures. |
| Source: Hydrogen data from a diagram in the review: John.
P. Hirth: "Effects of Hydrogen on the Properties of Iron and Steel", Metallurgical Transactions 11A (1980)
861 |
|
| |
| |
|
 |
What that means is that hydrogen at relevant concentrations will get into the liquid part
of the weld bead and from there will diffuse into the heat affected zone and beyond. When the structure cools down, hydrogen
will become supersaturated and, since it is highly mobile, always finds some defect where it can be absorbed. If that defect
is a small void or nanocrack - a dislocations pile-up might already do the trick - something new happens. Two
hydrogen atoms might find room in there, engage in sex and firmly bond, forming a hydrogen molecule (H2). This
liberates a lot of energy and thus will happen even if the crystal lattice has to be expanded a bit, i.e. even if the reaction
produces some elastic stress in the material. That stress attracts more hydrogen atoms, and pretty soon there will be a
nanocrack filled with hydrogen molecules that is under high internal pressure = stress.
Other hydrogen atoms may hang around dislocations, forming a kind of Cotrell-Bilby cloud. But in contrast to Cotrell-Bilby clouds formed by carbon or nitrogen interstitials, the
dislocation can never outrun its groupies in this case; they are just too mobile. Dislocations enveloped in a hydrogen cloud
thus will actually transport hydrogen over large distances as soon as they move in response to stress.
As soon as we
look into details of all the stuff related above, things become cloudy. While there is no lack of studies made and papers
written, there is still no full understanding of what exactly hydrogen does in iron (and other metals), and a lot of controversies
among the scientists involved. |
|
 |
When dealing with hydrogen it is best to forget everything about what interstitials normally
do. Hydrogen just is not normal. It can do a lot of things (in particular if your steel
contains all kinds of alloy elements and thus plenty of defects of all kinds), including a few special "quantum mechanical"
ones, since it is so small. For example, it might diffuse by "tunneling", something normal atoms never do. |
 |
The third question is the tough one. In the
relevant literature more than five different mechanisms for hydrogen promoted cracking are discussed. Some are mutually
exclusive. For example, suggestions have been made that hydrogen softens or hardens
iron /steel and that either mechanism somehow induces cracking.
It's time to point out that hydrogen-induced cracking
or hydrogen embrittlement is not confined to just welding iron or steel but
is a problem that plagues metallurgy in a rather broad context. Corrosion, for example, may produce hydrogen locally that
diffuses into the steel along grain boundaries and helps to cause the sudden catastrophical collapse of large structures
like the Berlin congress hall.
All
I'm going to do now is to look at some of the things that could happen in the context
of our basic equation for fracture: |
| |
|
|
| scrit |
» |
æ
ç
è |
2Y · g
pd |
ö
÷
ø |
1/2 |
< syield |
|
| Y = Young's modulus g = surface energy
of nanocrack, d = dimension of nanocrack, syield = yield strength
for plastic deformation |
|
| |
|
 |
What the equation says is that as soon as the critical stress is reached (at a
nanocrack tip), fracture will occur as long as the yield stress is higher than the critical stress. Hydrogen thus can promote
fracture by either lowering the critical stress and / or raising the yield stress. It will already be bad enough if that
happens only locally.
So let's see what kind of mechanisms could do that, and how mechanisms discussed in the literature
fit in with this concept |
|
 |
The Hydrogen Induced Decohesion Model says that dissolved
hydrogen reduces the cohesive strength of the lattice, i.e. the interatomic bond strength at a nanocrack tip and thereby
promotes decohesion. Since "interatomic bond strength" is just another word for Young's modulus - Check! Could work.
If the
hypothesis is true, the mechanism will bring down the critical stress for fracture. |
| |
 |
The Internal Pressure Model assumes that H2
formation in nanocracks, as pointed out above, produces high pressure inside these defects. The stress going with that is
part of the critical stress, which is thus reached for lower external stress. Check
once more! - could work. |
|
 |
The Surface Energy or Adsorption Model assumes that hydrogen
is adsorpted at newly generated surfaces and then reduces the surface energy. The critical stress then comes down - Check
once more! - could work. |
|
 |
The remainder of models deal with dislocations and hydrogen, and now it gets complicated.
I will not even try to discuss this. |
 |
Suffice it to say that cracking during and after welding is a rather complex matter.
To make things even more complicated, let's conceive that your weld did not develop cracks right away or after a few days.
Does that mean that you are home-free now? |
|
 |
Of course not. Cracks may still develop prematurely during re-heating, or in in creep or fatigue experiments. Or....
Well, if you are still with me and still considering to become a Material Scientist, congratulations! More timid persons
have long since deserted to simpler occupations like banking + tax evasion or, like A.
Merkel, to running a sizeable country. |
 |
We have covered a lot of ground by now. Have you noticed how cunningly I evaded
one of the more obvious questions concerning hydrogen-assisted cracking in welding? No? Well - here it is: |
|
 |
Why are those cracks typically found in the heat-affected zone,
quite often right at the interface to the formerly liquid part, see above?
Well - I
don't know. And neither, it seems, does anybody else. In all the papers and books that I consulted about this issue, the
topic was evaded. I could make an educated guess, and lots of researchers, no doubt, have already done this, but a simple
universally accepted explanation seems to be lacking.
The conclusion is obvious: |
| |
| |
| | |
There is nothing more practical than a good theory!
|
|
| |
| |
|
 |
We don't have a good welding theory yet. Let's hope that we will have one in the (near) future. |
| |
| |
© H. Föll (Iron, Steel and Swords script)




