|
If we were to use an epitaxial reactor for wafers covered with oxide, a layer
of Si would still be deposited on the hot surface - but now it would have no "guidance" for its orientation,
and poly-crystalline Si
layers (often just called "poly" or "polysilicon") would result.
|
|
 |
Poly-Si is one of the key materials in
microelectronics, and we know already how to make it: Use a CVD reactor and run a process similar to epitaxy. |
|
 |
If doping is required (it often is), admit the proper amounts of dopant gases. |
 |
However, we also want to do it cheap, and since it we want a polycrystalline layer,
we don't have to pull all the strings to avoid crystal lattice defects like for epitaxial Si layers. |
|
 |
We use a more simple CVD reactor of the furnace
type shown for oxide CVD, and we employ smaller temperatures (and low pressure, e.g. 60 Pa since we only
need thin layers and can afford lower deposition rates). This allows to use SiH4 instead of SiCl4;
our process may look like this: |
|
|
| | 60 Pa | |
| SiH4 | Þ |
Si + 2 H2 | | |
630oC | |
|
|
|
 |
Much cheaper! The only (ha ha) problem now is: Cleaning
the furnace. Now you have poly-Si all over the place; a little bit nastier than SiO2, but this
is something you can live with. |
 |
What is poly-Si used for and why it is a key material? |
|
 |
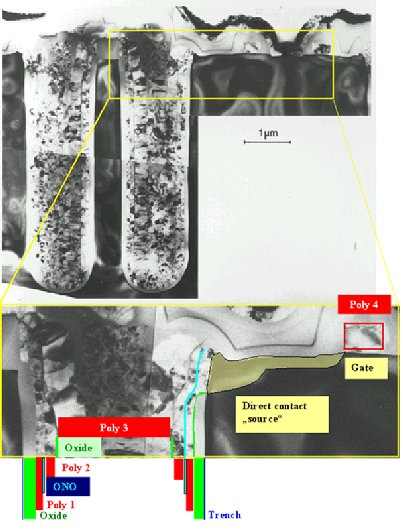
Lets look at a TEM
(= transmission electron microscope) picture of a memory cell (transistor and capacitor) of a 16 Mbit DRAM. For
a larger size picture and additional pictures click here. |
| |
|
 |
All the speckled looking stuff is poly-Si. If you want to know exactly
what you are looking at, turn to the drawing of this cross section.
We may distinguish 4 layers of poly Si: |
|
 |
"Poly 1" coats the inside
of the trench (after its surface has been oxidized for insulation) needed for the capacitor. It is thus one of the "plates"
of the capacitor. In the 4 Mbit DRAM the substrate Si was used for this function, but the space charge layer
extending into the Si if the capacitor is charged became too large for the 16 Mbit DRAM. |
|
 |
The "Poly 2" layer is the other "plate"
of the capacitor. The ONO dielectric in
between is so thin that it is practically invisible. You need a HRTEM -
a high resolution transmissin electron microscope - to really see it. |
|
 |
Now we have a capacitor folded into the trench, but the trench still needs to be filled. Poly-Si
is the material of choice. In order to insulate it from the poly capacitor plate, we oxidize it to some extent before the
"poly 3" plug is applied. |
 |
One plate of the capacitor needs to be connected to the source region of the transistor.
This is "simply" done by removing the insulating oxide from the inside of the trench in the right place (as indicated). |
|
 |
Then we have a fourth poly layer, forming the gates of
the transistors. |
|
 |
And don't forget: there were two sacrificial poly-Si
layers for the LOCOS process! |
 |
That makes 6 poly-Si deposition (that we know off). Why do we like
poly-Si so much? |
|
 |
Easy! It is perfectly compatible with single crystalline Si. Imagine using something
else but poly-Si for the plug that fills the trench. If the thermal expansion coefficient of "something else"
is not quite close to Si, we will have a problem upon cooling down from the deposition temperature. |
|
 |
No problem with poly. Moreover, we can oxidize it, etch it, dope it, etc. (almost) like single
crystalline Si. It only has one major drawback: Its conductivity is not nearly
as good as we would want it to be. That is the reason why you often find the poly-gates (automatically forming one level
of wiring) "re-enforced" with a silicide layer on top. |
|
 |
A silicide is a metal silicon compound, e.g. Mo2Si, PtSi, or Ti2Si,
with an almost metallic conductivity that stays relatively
inert at high temperatures (in contrast to pure metals which react with Si to form a silicide). The resulting double
layer is in the somewhat careless slang of microelectronics often called a "polycide"
(its precise grammatical meaning would be the killing of the poly - as in fratricide or infanticide). |
|
 |
Why don't we use a silicide right away, but only in conjunction with poly-Si? Because
you would loose the all-important high quality interface of (poly)-Si
and SiO2! |
|
| |
|
Si3N4
Deposition |
| |
|
 |
We have seen several uses for silicon nitride layers - we had LOCOS, FOBIC (and there are more), so
we need a process to deposit Si3N4 . |
|
 |
Why don't we just "nitride" the Si, analogous to oxidations, by heating the
Si in a N2 environment? Actually we do - on occasion. But Si3N4
is so impenetrable to almost everything - including nitrogen - that the reaction stops after a few nm.
There is simply no way to grow a "thick" nitride layer thermally. |
|
 |
Also, don't forget: Si3N4
is always producing tremendous stress, and you don't want to have
it directly on the Si without a buffer oxide in between. In other words: We need a CVD process for nitride. |
 |
Well, it becomes boring now: |
|
 |
Take your CVD furnace from before, and use a suitable reaction, e.g.
. |
| |
| 3 SiH2Cl2 + 4NH3 |
Þ |
Si3N4 + 2HCl + 1,5 H2 |
| | (» 700
oC)) | |
|
|
|
 |
Nothing to it - except the cleaning bit. And the mix of hot ammonia (NH3)
and HCl occurring simultaneously if you don't watch out. And the waste disposal. And the problem that the layers,
being under internal stresses, might crack upon cooling down. And, - well, you get it! |
| |
|
|
Tungsten CVD
|
| |
|
 |
For reasons that we will explain later, it became necessary at the end
of the eighties, to deposit a metal layer by CVD methods. Everybody would have loved to do this with Al -
but there is no good CVD process for Al; nor for most other metals. The candidate of choice - mostly by default
- is tungsten (chemical symbol W for "Wolfram"). |
|
 |
Ironically, W-CVD comes straight form nuclear power technology. High purity Uranium
(chemical symbol U) is made by
a CVD process not unlike the Si Siemens process using UF6
as the gas that decomposes at high temperature. |
|
 |
W is chemically very similar to U, so we use WF6 for W-CVD. |
 |
A CVD furnace, however, is not good enough anymore. W-CVD needed
its own equipment, painfully (and expensively) developed a decade ago. |
|
 |
We will not go into details, however. CVD methods, although quite universally summarily
described here, are all rather specialized and the furnace type reactor referred
to here, is more an exception than the rule. |
|
| |
|
Advantages and Limits of CVD Processes
|
| |
|
 |
CVD processes are ideally suited for depositing thin layers
of materials on some substrate. In contrast to some other deposition processes which we will encounter later, CVD
layers always follow the contours of the substrate: They are conformal to the substrate as shown below. |
| |
|
 |
Of course, conformal deposition depends on many parameters. Particularly important
is which process dominates the reaction: |
|
 |
Transport controlled process
(in the gas phase). This means that the rate at which gas molecules arrive at the surface controls how fast things
happen. This implies that molecules react immediately wherever they happen to reach the hot surface. This condition is always
favored if the pressure is low enough. |
|
 |
Reaction controlled kinetics. Here a molecule may hit
and leave the surface many times before it finally reacts. This reaction is dominating at high pressures. |
 |
Controlling the partial pressure of the reactants therefore is a main process
variable which can be used to adjust layer properties. |
|
 |
It is therefore common to distinguish between APCVD (= atmospheric pressure CVD) and
LPCVD (=
low pressure CVD). |
|
 |
LPCVD, very generally speaking, produces "better" layers. The deposition
rates, however, are naturally much lower than with APCVD. |
 |
CVD deposition techniques, though quite universal and
absolutely essential, have certain disadvantages, too. The two most important ones (and
the only ones we will address here) are |
|
 |
They are not possible for some materials; there simply is no suitable chemical reaction. |
|
 |
They are generally not suitable for mixtures of materials.
|
 |
To give just one example: The metallization layers for many years
were (and mostly still are) made from Al - with precise additions of Cu and Si in the 0,3% - 1%
range |
|
 |
There is no suitable Al-compound that decomposes easily at (relatively low) temperatures.
This is not to say that there is none, but all Al-organic chemicals known are too dangerous to use, to expensive,
or for other reasons never made it to production (people tried, though). |
|
 |
And even if there would be some Al CVD process, there is simply no way at all to incorporate
Si and Cu in the exact quantities needed into an Al CVD layer (at least nobody has demonstrated it
so far). |
 |
Many other materials, most notably perhaps the silicides, suffer from similar
problems with respect to CVD. We thus need alternative layer deposition techniques; this will be the subject of the
next subchapter. |
| |
|
| Footnote: |
The name "Poly
Silicon" is used for at least three qualitatively very different kinds of materials: |
|
1. The "raw material" for crystal growth,
coming from the "Siemens" CVD process. It comes - after breaking
up the rods - in large chunks suitable for filling the crucible of a crystal grower. |
|
2. Large ingot of cast Si and the thin sheets made
from them; exclusively used for solar cells. Since the grains are
very large in this case (in the cm range), this material is often referred to as "multi
crystalline Si". |
|
3. The thin layers of poly Si addressed
in this sub-chapter, used for micro electronics and micro mechanical technologies. Grain sizes then are µm or less. |
| |
In addition, the term poly Si might be used (but rarely is) for the dirty
stuff coming out of the Si smelters, since this MG-Si
is certainly poly-crystalline |
© H. Föll (Electronic Materials - Script)