|
The abbreviation "LOCOS" stands for "Local Oxidation of Silicon" and was almost a synonym for MOS devices, or more precisely,
for the insulation between single transistors. LOCOS makes the isolation between MOS transistors considerably
easier then between bipolar transistors, cf. the drawings discussed before: |
|
 |
For bipolar transistors,
you have to separate the collectors. This involves an epitaxial layer and some deep diffusion around every transistor. |
|
 |
For MOS transistors, no isolation
would be needed weren't it for the possible parasitic transistors. And this problem can be solved by making the "gate
oxide" of the parasitic transistors - which then is called field oxide - sufficiently
thick. |
 |
The thick field oxide has been made by the LOCOS process from the beginning
of MOS technology until presently, when LOCOS was supplanted by the "box
isolation technique", also known as "STI" for "Shallow
trench isolation". |
|
 |
Since the LOCOS technique is still used, and gives a good example of how processes
are first conceived, are optimized with every generation, become very complex, and are finally supplanted with something
different, we will treat it here in some detail |
 |
As the name implies, the goal is to oxidize Si only locally,
wherever a field oxide is needed. This is necessary for the following reason: |
|
 |
Local (thermal) oxide penetrates into the Si (oxidation
is using up Si!), so the Si - SiO2
interface is lower than the source - drain regions to be made later. This
could not be achieved with oxidizing all of the Si and then etching off unwanted oxide. |
|
 |
For device performance reasons, this is highly beneficial, if not absolutely necessary. |
 |
For a local oxidation, the areas of the Si
that are not to be oxidized must be protected by some material that does not allow oxygen
diffusion at the typical oxidation temperatures of (1000 - 1100) 0C. We are talking electronic materials
again! |
|
 |
The
only material that is "easily" usable is Silicon
nitride, Si3
N4. It can be deposited and structured without too much problems and it is compatible with Si.
|
|
 |
However, Si3
N4 introduces a major new problem of its own, which can only be solved by making the process more complicated
by involving yet another materials. This gives a succinct example of the statement
made before: That materials and processes have to be seen as a unit. |
|
 |
Lets see what would happen with just a Si3
N4 layer protecting parts of the Si from thermal oxidation. |
| |
|
|
 |
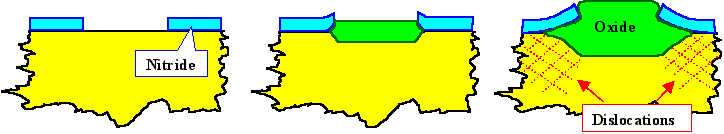
Oxygen diffusion through the oxide already formed would also oxidize the Si under the Si3
N4; i.e. there would be some amount of lateral oxidation. Since a given volume of Si expands
by almost a factor of 2 upon oxidation (in other words: Oxidizing 1cm3 of Si produces almost
2 cm3 of SiO2), the nitride mask is pressed upwards at the edges as illustrated. |
|
 |
With increasing oxidation time and oxide thickness, pressure under the nitride mask increases,
and at some point the critical yield strength of Si at the oxidation temperature
is exceed. Plastic deformation will start and dislocations are generated and move into
the Si. Below the edges of the local oxide is now a high density of dislocations which kill the device and render
the Si useless - throw it out. |
|
 |
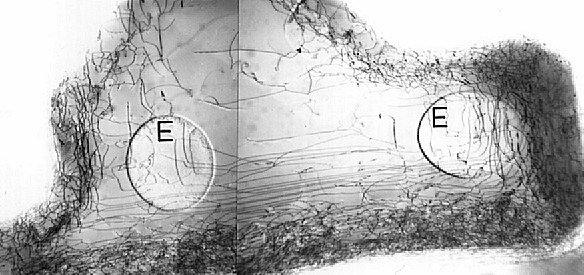
This is not "theory", but eminently practical as shown in the TEM
picture from the early days of integrated circuit technology: |
| |
|
|
 |
We are looking through a piece of Si. The dark lines are the projections of single
dislocations, the "dislocations tangles" corresponds to oxide edges; "E" shows contact areas
(emitters) to the Si. Another picture can be found in the link. |
|
 |
Actually, it doesn't even need the oxidation to produce dislocations. Si3
N4 layers are always under large stresses at room temperature and would exert great shear stresses
on the Si; something that can not be tolerated as soon as the nitride films are more than a few nm thick.
|
 |
We arrive at a simple rule: You cannot use
Si3
N4 directly on Si - never ever. What are we to do now, to save the concept of local oxidation? |
| |
|
Buffer Oxide |
| |
|
 |
We need something between the Si3
N4 mask and the Si; a thin layer of a material that is compatible with the other two and that
can relieve the stress building up during oxidation. Something like the oil in you motor,
a kind of grease. |
|
 |
This "grease" material is SiO2, as you might have guessed - it
was already mentioned before under its proper name of "buffer
oxide". The hard Si3
N4 (which is a ceramic that is very hard not yielding at a "low" temperature of just about
1000 oC), is now pressing down on something "soft", and the stress felt by the Si will
not reach the yield stress - if everything is done right. |
|
 |
The situation now looks like this |
| |
|
|
 |
No more dislocations, but a comparatively large lateral oxidation instead, leading to a configuration
known as "birds beak" for the obvious reason shown in the picture to the right
(the inserts just are there to help you see the bird). |
 |
So we got rid of one problem, but now we have another one: The lateral extension
of the field oxide via the birds beak is comparable to its thickness and limits the minimum feature
size. |
|
 |
While this was not a serious problems in the early days of IC technology, it could
not be tolerated anymore around the middle of the eighties. |
|
 |
One way out was the use of a poly-Si layer as a sacrificial layer. It was situated
on top of the buffer oxide below the nitride mask and was structured with the mask. It provided some sacrificial Si
for the "birds beak" and the total dimension of the field oxide could be reduced somewhat. |
|
 |
This process is shown in comparison with
the standard process in the link. |
 |
But even this was not good enough anymore for feature sizes around
and below 1 µm. The LOCOS process eventually became a very complicated process complex in its own right;
for the Siemens 16 Mbit DRAM it consisted of more than 12 process steps including: |
|
 |
2 oxidations, 2 poly-Si deposition, 1 lithography, 4 etchings
and 2 cleaning steps. |
|
 |
It was one of the decisive "secrets" for success, and we can learn a simple truth
from this: |
 |
Before new materials and processes are introduced, the existing materials and
processes are driven to extremes! And that is not only true for the LOCOS process, but for all other processes.
|
|
 |
Still, with feature sizes shrinking ever more, LOCOS reached the end of its useful
life-span in the nineties and had to be replaced by "Box isolations",
a simple concept in theory, but hellishly difficult in reality. |
|
 |
The idea is clear: Etch a hole (with vertical sidewalls) in the Si wherever you want
an oxide, and simple "fill" it with oxide next. More about this process can be found in the link above. |
© H. Föll (Electronic Materials - Script)