|
10.2.4 Bloomeries |
| |
Real Smelting |
 |
So you smelted some iron. Some time between 1000 BC and 1000 AD or even later.
It doesn't matter much as far as the basics are concerned. You used a "bloomery"
type of furnace, essentially a stone lined hole in the ground plus a cylindrical tube made from e.g. clay on top; see all
the pictures below and elsewhere. A bellows is attached via a tuyere. Your bloomery is not much different from the shaft
furnaces used for making copper.
You did the best you could, trying to follow the working recipe as closely as possible.
You and your crew worked for many hours. Some roasted the ore and smashed it into small pieces of suitable size; others
built the smelter and the hearth of the forge. During smelting time it's working the bellows, feeding the smelter, fetching
the beer, and watching closely if everything goes well. You may tap the slag once in a while through a tap hole or you just
let it collect in the bottom pit. |
|
 |
Now it is time to stop the smelting. How do you do this? You tell the guy at
the bellows to stop pumping? Then your smelter stack will be full of burden that got stuck when the fire goes out and everything
cools down. That will be no problem if you tear down the whole thing to get at the bloom while it is still hot. It's just
wasteful.
So you stop feeding the smelter but keep blowing air until everything is burned off. Then you use up all material.
It's just that the smelting process sort of fizzles out uncontrolled during this turning-down process and you don't know
what happens to your bloom. But you might be able to get your bloom out through the top or an opening at the bottom without
being forced to tear down your smelter completely. | |
|
| |
 |
Here are some pictures of a smelting experiment. It was re-enacted
to investigate the old techniques and also to see what one would dig up years later: |
| |
| |
| |
 |
| Small Bloomery starting up, dismantled, and bloom |
Source: Thomas Birch, Robert Scholger, Georg Walach, Frank Stremke,
Brigitte Cech: "Finding the invisible smelt: using experimental archaeology to critically evaluate fieldwork methods
applied to bloomery iron production remains", Archaeol Anthropol Sci; Published online 16. Mai 2013.
With friendly
permission |
|
| |
| |
|
 |
This was a smelting experiment done by archaeometallurgists. That's why we have
data and numbers:
- The ore was limonite, roasted and then crushed and sorted.
- 15 kg charcoal for preheating.
- Constant airflow until the end stages of the smelt, when it was increased.
- 18 kg ore, 45 kg charcaol; ore to fuel ratio 1 : 2.5.
- Charge added every 20 min.
- The slag-rich bloom weighed 9.5 kg
| |
|
| |
| |
| |
 |
This was high-efficiency smelting. Limonite contains about 50 % iron
so at most 9.5 kg iron could be produced. Even if only halve of the bloom consists of iron this is a good result; compare
to numbers given here.
More pictures of blooms and bloomeries can be found in the link.
|
|
 |
Note that no flux was added. This implies that there was sufficient
SiO2 in the gangue. Slag from smelting iron invariably contains fayalite or iron
silicate (Fe2SiO4), a member of the olivine group that constitute the Earth's most common minerals
by volume. SiO2 or quartz reacts with iron oxides in many ways; the end products being fayalite, wüstite, the iron mono-oxide FeO rarely found in nature, some elemental
iron (Fe) plus carbon mono and di-oxides. What, exactly, you get depends on conditions; here are a few possibilities for
the end result of all interesting reactions: |
| | |
|
| |
| 1. | 3 Fe2O + 2 SiO2 + 3 CO |
Þ |
2 FeO + 2 Fe2SiO4 + 3 CO2 |
| 2. | 2 Fe2O + SiO2+ 3 CO |
Þ |
Fe + FeO + Fe2SiO4
+ 3 CO2 | | 3. |
4 Fe2O + SiO2 + 9 CO |
Þ |
5 Fe + FeO + Fe2SiO4
+ 9 CO2 | | 4. | 2 FeO + SiO2 |
Þ |
Fe2SiO4 + 909 kJ |
|
| |
| |
|
 |
About equal amounts of wüstite and fayalite, i.e. 2
FeO + 2 Fe2SiO4 plus a bit of this and that is what we call slag from bloomery iron smelting.
It is liquid around 1200 oC (2192 oF).
The reactions above have been made "stoichiometric"
for certain relations of iron and SiO2 gangue in ore. Since the fayalite forming process releases a lot of energy,
far more than the other processes in the smelter (see the last entry above), it will definitely take place and elemental
iron can only be produced if some iron oxide is left over after all the the SiO2 in the gangue has reacted. The
reactions given above (and taken from Vagn Buchwald's
book) correspond to the following iron : SiO2 relations in the ore:
- Fe : SiO2=56 % : 20 %. An inadequate ore that will only produce slag.
- Fe : SiO2=59 % : 16 %. The iron yield is 25 % relative to the iron fed into the smelter-
- Fe : SiO2=64 % : 8.6 %. The iron yield is now a very high 62.5 %.
|
 |
This is quite amazing! Change your ore "quality" by a few percent and
your smelting results may change dramatically. That's why you paid a lot of attention to your ore. You cleaned it as well
as possible, you crushed it and then roasted it. That process can be seen
here. Roasting takes place around 500 oC (932 oF) for several hours and does a number of things:
- It removes water. Both the "regular" water and the crystal
water in the ore by reactions like 2 FeOOH Þ Fe2O3 + H2O.
In other words; it turns Goethite, limonite
and bog iron into haematite.
- It removes sulfur (S). Many ores contain some pyrite (FeS2).
Roasting releases the sulfur according to 4 FeS2 + 11 O2
Þ Fe2O3 + 8 SO2. The sulfur dioxide produced is a poisonous
gas that escapes and will be noted by its acrid smell. Since sulfur in iron is always bad news, this is quite important.
- It makes the ore more crumbly because the iron oxide crystals will fall apart to some extent undergoing all these reactions.
This increases the surface to volume ratios substantially and thus makes the solid-state reduction process easier that can
only proceed via the surface.
- It changes the color of the iron-bearing parts (bright orange if it is pure haematite). That makes picking of good ore
pieces now easier. Some unwanted additions like copper may cause discolorations that allow to discard the contaminated pieces.
So you did a lot of work before you started to actually smelt. |
 |
Anyway, the smelter is is now turned off one way or other and now it is time to
get the bloom. That is not a problem as long as the smelter was nothing but a hole in the ground about 30 cm across, lined
with a few stones, and a clay tube leading up about 1 m at most. You just tear down the whole thing. You can build a new
one in a short time with little cost and effort. The tuyere, the bellows and the connection between both are more critical.
You could also make make a bigger smelter but then tearing it down and rebuilding gets more expensive. More important:
the bloom might be too large to be wrought (=old-fashioned word for worked) "by
hand". A bloom weighing more then - very roughly - 10 kg needed more bang for compacting than what you and I could
deliver with a hammer.
The smelter shown above did have an opening for retrieving the bloom (plugged up during operation)
but since it was too small, the smelter had to be dismantled. You can try to retrieve the bloom through the top of the stack
but it ain't easy either. All in all, iron bloomeries for more than 2000 years tended to be small and were only used once
or - after repairs - a few times for good reasons. |
|
 |
Metal engineers eventually, like after 1000 AD, found ways to have larger permanent
smelters and to get the bloom out without destroying the smelter every time. They also found ways to work those big blooms
- with water-wheel driven hammers! But we are still back around 1000 BC and we need to wait for about 2000 year before that
is going to happen.
The best one could do for more than 2000 years was to produce a bloom about 2 to 4 times bigger
than what one man could work. The big bloom then was cut into two or maybe four parts while still red hot. Then up to four
smiths could work it. Here is a picture showing a cut bloom.
|
| | |
|
| |
Working your Bloom |
 |
So what, exactly, is a bloom, and how do I work it? As the pictures in the links
show, a bloom is a red-hot mass of iron, charcoals, slag, and maybe pieces of smelter lining, weighing a few kilograms.
If it is larger than roughly 10 kg, you must divide it. That is easier said then done, especially without modern power tools. |
|
 |
You must now compact and refine your bloom. For that you hit it with a hammer. For starters, you need a hammer with a big head
otherwise you just make dents in the red-hot porous mass that is oozing viscous slag. That's why you use a wooden mallet
and a stone or the slightly concave surface of a tree trunk as anvil. It's not that wood makes a great hammer head or anvil.
But if you ever tried to lift an iron hammer the size of the wooden mallet shown below,
you appreciate the wooden mallet. Same thing for the tree trunk. It will get burned but keeps the bloom nicely in place.
|
| |
| |
| |

| | Ready to compact a bloom |
Bad bloom from Roman times |
Source left
Source right: From the "Hüttenberg" project under the direction of Brigitte
Cech; photo from Ruth Fillery-Travis; both University College London (UCL). With friendly permission.
|
|
| |
| |
 |
What you want to do is to hammer your bloom into an solid piece of "wrought" iron. Wrought because you wrought=worked it, not
necessarily because it had a very low carbon content. This means that you must remove bits and pieces of foreign matter,
squeeze out the still liquid (if viscous) slag, and close the holes and pores in the iron.
This is hard work, necessitating
a decent supply of beer. While I have never yet hit a bloom with a mallet as shown above, I do have some hands-on experience
of hitting things with big wooden (or iron) hammers for prolonged periods of time, and I can testify that it is hard work,
producing a magnificent thirst that cannot be quenched with water.
Hitting a bloom with a wooden mallet will only get
you that far, though. At some point the bloom is just too cold to notice your feeble hammer blows. You now transfer it to
the hearth of a smithy, heating it up again, and banging it some more. This you do on an anvil (stone in ancient times),
using an iron hammer. In between banging you reheat the piece in your hearth. You go for very high temperatures, necessitating
a good air supply to you hearth. Very high temperatures in excess of 1000 oC (1832 oF) make it not
only easier to shape your piece, but re-liquefy still present slag and enable fire-welding of the many seams. |
|
 |
Just banging on your bloom randomly will compact it some but you will not achieve optimal
results. It takes some experience and cunning to produce a nice bar of iron (or shapes like Celtic double-pyramid bar; see
below) from a given bloom. You will, for example, sprinkle some special sand on the mass from time to time, because that
helps to hammer or fire weld iron to iron.
If you want to close holes, you must join iron surfaces, and this is not possible as long as they are oxidized. Adding a
bit of silicate (from quartz sand) will produce liquid fayalite when it
reacts with the oxide, the main material in slag, that you now can squeeze out. I'll get to that in more detail in a moment.
You also will tend to fold your flattened piece over and weld it together, and you repeating that several times. In other
words, you produce what some like to call a piece of "damascene" iron / steel. Well - you don't. All you try to
do is to make your piece of iron as uniform as you can, and folding and rewelding a lot does just that. Done professionally
we call it "faggoting". Try it with random lumps
of brown (chocolate) and white dough. Flatten, fold and "weld" a few times and you have more uniform light-brown stuff.
|
 |
Of course, the quality of a bloom matters very much in this. Some blooms are simply
too bad for "processing"; and experts can "see" that, it appears, and won't bother to work it. The "bad"
bloom above was found in Hüttenberg; Austria, one of the places where the the famous
"Ferrum Noricum" of the Romans was produced
between 100 BC - 400 AD. It was found when the smelting place was dug up by Brigitte Cech and others like Thomas Birch.
We must assume that it was judged to be unworthy of use by the ancient smiths. |
|
 |
Here is a bloom from a modern experiment, it looks rather similar to the "bad" Roman
bloom from above. |
| |
| |
| |

| | Bloom from a recent experiment |
| Source: Besucherbergwerk „Grube Wohlfahrt“ |
|
| |
| |
 |
When the smith is done, he has produced a shapely piece of iron. A straight bar,
perhaps, or the famous Celtic "double pyramid" bars (Spitzbarren)
as shown below: |
| |
| |
| |

|
"Double pyramid" iron bars found in my home town in 2009
Typically made
from two blooms; the (bad) welding line is visible. |
| Source: Photographed at the "Special
Exhibition Dedicated to the Celts of the First Millennium BC"; Stuttgart, 2012/13 |
|
| |
| |
|
 |
But no matter how good your bloom and your compacting skills: the iron / steel pieces produced
contained slag particles and maybe other inclusions, and their carbon content varied within a piece and between pieces (as
we will see in the next chapter). They may or may not contain relevant (varying) concentrations of phosphorous and other
stuff, too. Moreover, closing holes in the bloom and folding the workpiece many times produced plenty of weld seams that
can be perfect or bad, depending on the skills of the smith and local conditions. The specimen above, for example, show
"cracks", probably from bad weld seams.
This kind of stuff can never be
a match for inclusion-free homogeneous steel! |
| | |
|
| |
The Carbon Content of Your Bloom |
 |
Now I can no longer avoid dealing with the crucial question: What did those bloomeries
really produce? Always wrought iron (=soft iron with very low carbon content) as explicitly
and implicitly assumed in most of the older "archeological" papers? And never cast iron?
In the preceding
sub-chapter I have already argued at length and rather convincingly (to me) that it is possible to produce low carbon iron
in a bloomery but that you just as well could get cast iron or anything in between, i.e. all grades of carbon steel. It
now behooves me (love that word) to prove that claim. There are two possible ways to do that: |
| |
- Analyze a lot of old iron and determine the carbon content, in particular for everyday "wrought iron" stuff.
- Run bloomery experiments just as it was done thousands of years ago and see what you can get.
It is quite nice for me that the number of bloomery experiments done by all kinds of people and thus also by people
who don't know a thing about Material Science is sky rocketing! Before the year 2000 or so, only a few smelting experiments
were done inside the scientific community, e.g. by Tylecote.
Nowadays lots of people with or without a scientific background go out, build a bloomery, drink some beer and smelt some
iron. Even high school classes do it for their science projects. I'm not sure if they drink a lot of beer and I don't even
want to know how they keep themselves amused otherwise out there in the underbrush. |
|
|
| |
 |
This is good! The kids and many enthusiasts out there do not know a thing about
smelting except what they have heard (or read) from others - just like the old Romans and so on. Many a hobby black smith
or metal smelter started with very little background knowledge but became a smelting expert by trial and error, who could
raise a bloomery and produce some good iron /steel without problems.
It's a rare moment! We also get to witness the
errors in the present wild days of empirically re-inventing bloomery techniques by trial and error! My guess will be that
in a few years it is all over. The Internet will provide so many (almost) fail-proof recipes for smelting a bit of iron
that will work all the time. Just like in antiquity; it just took a bit longer then for the knowledge to spread. |
 |
The link above gives some results from randomly selected smelting activities.
A clear picture emerges:
- It is perfectly possible to smelt cast iron, wrought iron, and anything
in between in a simple bloomery.
- It is also possible to smelt no iron, producing only iron-rich ("magnetic") slag.
- Efficiencies (weight of bloom relative to weight of ore) can vary substantially; high efficiencies are possible.
- Changing the carbon content of the bloom by changing parameters "by feeling" doesn't seem to work most of
the time.
- Bloom quality can vary a lot, and the carbon content of a bloom can change considerably from top to bottom.
- It does take a fairly large number of smelting experiments done in the time honored trial and error method before you
can call yourself an experienced smelter.
- It is possible, however, to develop a smelting technique that produces what you want (i.e. low-carbon iron) most of
the time within the possibilties and constraints of your local environment.
- It's not so easy to transfer a technology that works in one place to another one where the ore, charcoal and so on will be somewhat different
|
 |
Now let's look at the archeological record. Below I give carbon concentrations
for some objects as given and discussed in Buchwald's authoritative book.
|
| |
| |
| |
Iron clamps Parthenon
447 BC |
Roman nails
40 AD |
 |
No | [C %] |
No | [C %] |
 |
| 1 | 0.395 |
1 | 0.019 |
| 2 | 0.419 |
2 | 0.21 |
| 3 | 0.117 |
3 | 0.04 |
| 4 | 0.104 |
4 | 0.07 |
| 5 | 0.350 |
| |
Left: Iron stub for connecting column drums in-situ in Ephesos
Right:
Roman "standard" nail |
|
| |
| |
|
 |
The Greek routinely used iron clamps (always encased in lead) to keep layers of
shaped stones together, and stubs as shown between two column drums. This added resistance to shear forces as they might
be encountered in an earthquake. The clamps that kept the marble "bricks" of the temples together sure varied
a lot with respect to their carbon concentration. None was "wrought iron" in a strict sense (i.e. less than 0,1 % of carbon). That makes sense. You want strong steel
to make your temples earthquake-prove. I have my doubts, however, that the Greeks knew much about the properties of their
iron / steel.
Nails you typically like to be made from soft and thus very bendable iron. It thus makes sense that the
Roman nails are low in carbon. Three of them would even qualify as wrought iron.
Now let's look at some Celtic swords from around 350 BC. They
all have been investigated in detail by R. Pleiner.
Vagn Buchwald was able to re-examine 23 of these swords, including the structure and compositions of the slag inclusions.
His results concerning the carbon and phosphorous concentration are shown below in a graphic representation drawn with the
data in Buchwald's book. The carbon concentration
typically varies between different parts in an unsystematic way and the measured range is shown as red bar. The phosphorous
values represents the maximum concentrations found. |
| | |
|
| |
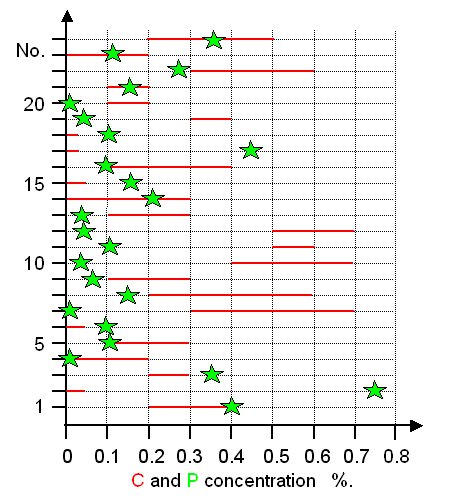 |
| Carbon (C) and phosphorous (P) concentrations in Celtic swords from about 500 BC - 250
BC. |
| Source: Data are from Buchwald's
book; p. 116 |
|
| |
| |
|
 |
There are four wrought iron swords, rather
useless one should think. But two of them are high in phosphorous and thus also quite hard. The rest represents a mixture
of all kinds of carbon concentrations - between swords and in a given sword. |
 |
Rehder in his
wonderful book raises the same point. Bloomeries produce iron with a wide spectrum of carbon concentrations. He analyzed
data for 507 iron artifacts, spanning the time range from 1000 BC to 500 AD, and, after eliminating cast iron ("which
was so easily made") arrived at an average carbon concentration of 0.48 % with a range from 0.001 % to 0.9 %. Rehder
also reports on an independent work of Schaaber et al. where an average carbon concentration of 0.53 % was found for 484
samples, mostly from late antiquity in central and southern Europe. |
|
 |
Rehder also makes a point that finished iron objects had experienced
a lot of forging that must have lead to some de-carburization. I have discussed at
length that carburization during forging is not impossible but unlikely and that not much will happen in any case. De-carburization
is far more likely, since most parts of the forged objects will experience oxidizing conditions most of the time - and that's
when de-carburization takes place. It also will only affect surface-near parts, but with a lot of folding and fire-welding,
the effect might be noticeable throughout the volume. |
| |
| |
| |
What's Left |
 |
Untold millions of bloomeries must have been used in Europe and the Middle East
in the more than 2500 years between 1200 BC and 1500 AD. It shouldn't be too difficult to dig some up. What can we expect
to find? |
|
 |
Not much exiting stuff, actually. Besides a (completely grown over) slag
heap you can only expect some burnt looking stones and discolored soil wherever there has been a hole-in-the-ground
bloomery. With luck, maybe some pieces of the wall and the tuyere are still in-situ. With extreme luck, you might find an
abandoned bloom. Archeometallurgists actually have run bloomeries with all the trimmings (roasting the ore, forging the
bloom, ...) for a while. Then they tore down the smelter, cleaned up a bit, and went away. |
| |
| |
| |
|
| |
| |
|
 |
After just a few years grass will have grown over the whole affair and nobody
will recognize that the place was used for smelting some iron some time ago. After a few thousand years, some topsoil has
aggregated and nothing is visible at all - by human eyes. With some special "eyes" provided by your friendly physicist
or Materials scientist around the corner that can also "see" (very small) magnetic fields, you will recognize
something special there, however.
When you dig it up, it might look like this: |
| |
| |
| |
 |
| Remains of a Roman furnace in Hüttenberg, Austria |
| Source |
|
| |
| |
 |
Not much is left from old bloomeries and forges. Essentially
you find some holes in the ground or just depressions with some blackened soil. Maybe a few stones and some slag pieces
if you just take in an overview. If that site is from 1000 BC or 1000 AD is not immediately obvious; the remains of smelting
places look very much the same, regardless of age. If you dig and
look closely, however, you find much more:
- Slag deposits from the "run-off" slag (Run slag, tap slag, ...) if the furnace was tapped for slag.
- Furnace bottom slag (furnace bottom cakes) if there was no tapping and the slag collected a the bottom.
- Hearth slag. That is slag released when the bloom is re-heated in the hearth. It trickles down and leaves a "cake"
looking very much like furnace bottom slag.
- Hammerscale or oxide particles that flew off when the smith banged on the always oxidizd hot iron.
- Pieces of iron banged off from blooms and the hot iron parts.
- Discarded "bad" blooms.
- Hammer and anvil stones.
- Furnace lining stones.
- Remainders of flux and ore.
- "Lost" items like an iron bar or beer bottles from more recent smelting.
No. 5 - 10 are obvious, and I won't discuss them. The first four items, however, warrant a few words (and pictures). |
 |
For
finding the "run-off" or production slag, you may want to look around
yourself. Then consider that those hillocks and the large hills you see out there might be huge mounds of slag! We have
seen that before in the context of copper smelting. Now
we may encounter really big deposits of slag. We definitely do! |
|
 |
In 1915 - 1943 the modern steel mills on the Island of Elba (Portoferraio)
or just across in Italy (Populonia, Follonica, Piombino) were fed with iron-rich ancient slag from the general area. Up
to 2 million tons of ancient slag, found in layers 2 m - 8 m thick, were "harvested"
with heavy equipment. The slag was produced by bloomeries running there for a long time, the maximum activity was after
450 BC. Ancient slag from bloomeries always contains a lot of iron because iron oxide (=ore) was used as flux. Modern blast
furnaces use limestone as flux (producing CaSiO4 instead of FeSiO4) and thus can work with old slag.
True, the general area (including the Island of Elba) was a metallurgical center in antiquity (sort of an ancient Pittsburg,
Sheffield or "Ruhrgebiet") for many hundreds of years - but 2 million tons?
Assuming that one bloomery produces at most 50 kg of slag in one run,
this means that 40 million
bloomery runs must have taken place. Assume somewhat smaller numbers, and it still boggles the mind.
So
we could expect that the archeologists dug up a lot of bloomery remains and lots of artifacts related to iron making? Not
so! First, the large strip-mining machinery left little behind, and second, the archeologists then did not care all that
much about blackened holes in the ground. They cared about the discovery of unrobbed Etruscan
graves around Populonia from about 900 BC - 420 BC that were hidden (and thus protected) below thick layers of slag.
The Etruscians were about in Italy before and during the rise of the Roman empire and
masters of copper / bronze technology. They were also easy going, given to throwing good parties (look at the pictures in
those tombs), and not above making fun of their victims: |
| | |
|
|
|
 |
Etruscantomb ("tumuli") at Popolunia; formerly hidden
under slag.
Inset: Embellishment on a chariot. Last thing you might have seen while still alive. |
| Source: Internet commercials |
|
| | |
|
|
 |
In the graves some iron objects were found but few have been analyzed. As far
as one can tell, the old Etruscian iron was, as expected, a mix of all kinds of grades. |
 |
Furnace bottom slag and
hearth slag look rather similar. In the first case it is the stuff that collected at the
very bottom of the bloomery and couldn't flow out because the tap hole was too high (or because there was no tap hole).
In the second case it is the slag that drips down from the bloom when it is re-heated to be forged to its final shape. Forging
sqeezes out the slag still in the bloom and possibly adds a bit more because the smith sprinkles silica on the bloom. Some
of the stuff clings to the bloom and only flows off during reheating.
Furnace bottom slag and hearth slag thus will
be roundish and bowl-shaped on one side. |
|
 |
Here is a 100 ton furnace slag found in Rochester, USA, in 2013, when the City
dug up a parking lot in order to move some electrical lines in preparation for the construction of Rochester’s new
marina. It so happens that in 1926 or so, the Quinnesee Iron Mining Co. operated a blast furnace right there, but when they
folded and tore down the furnace, they forgot to take the rather weighty furnace bottom slag along. |
| | |
|
| |
|
 |
| 100 ton (about 220 000 pounds) furnace bottom slag in Rochester, USA. |
| Source: "Rochester Subway". |
|
| | |
|
|
 |
The furnace bottom slag of an ancient bloomery looks about the same, except it
is just much smaller.
Here is a typical hearth slag, cut into two pieces. It has the same basic geometry as a furnace
bottom slag because both once filled a round bowl-shaped depression. |
| | |
|
| |
|
 |
| Hearth slag . |
| Source: English Heritage Internet site. With permission. |
|
| | |
|
|
 |
There is no lack of ancient slag and analyzing it has become a major activity.
Slag, however is always a complex mixture of many ingredients; here
is an example. It is therefore not easy to learn a lot about the smelting and forging process from slag analysis alone.
However, much progress is being made right now and the analysis of slag will certainly provide major insights into ancient
metallurgy in years to come. |
 |
Now to hammerscale, the last point of the list above
that I will discuss. What is hammerscale? It is the oxide formed on your iron when you put it into the forge fire. You then
simply "burn" it. When you bang it with the hammer, the brittle oxide breaks off in small fish-scale like parts.
Small iron particles might break off too, becoming oxidized as the fly off, and if your iron still contains some slag, droplets
of liquid slag, solidifying as the sail off, will join the rest.
Here is a picture of hammerscale on the anvil: |
| | |
|
|
|
 |
| Hammerscale on an anvil. |
| Source: Sorry, forgot. |
|
| | |
|
| |
 |
Hammerscale is essentially Magnetite (Fe3O4). Quite a bit
is produced during forging and that limits the time you can heat and bang a piece of iron. It gets smaller all the time.
Hammerscale is a great ore. If you produce enough you can put it right back into your smelter. |
 |
Enough! It should have become quite clear that iron and steel was
mostly made in small bloomeries for more than 2000 years. The product always contained slag inclusions, and carbon (and
other stuff) in all kinds of concentrations. With experience and cunning, the process could be optimized to some extent
but the final product could never compete with uniform iron / steel that did not contain slag inclusions, meaning steel
that was liquid once. |
| |
 |
It remains to see how Europe and some of the East cultivated bloomers iron /
steel technology and how in the far East wootz steel came into being, a steel that was liquid once and thus free of slag
inclusions. |
| | |
|
© H. Föll (Iron, Steel and Swords script)











