|
6.2.3 Welding with Hammer and Fire |
 |
All the old sword blades we marvel at in museums or books have not been cast. Some welding may have been involved, though. We thus must
consider welding to some extent. We will do that right here and then come back to it in the context of making swords. |
|
 |
Welding for most people means to join two metals of the same kind by filling the gap between the metal pieces with liquid metal
of the same kind. You do it be pressing the two pieces together and heat the seam until
things melt. In addition, you may feed some material to the seam.
Whatever you do, parts of the metals to be joined
will be liquefied too to some extent. Let's call that kind of welding liquid
welding. |
 |
By the way, if we join two metals by filling the gap between the two with a liquid
metal of a different kind that has a far lower melting point, we call that process soldering. And by the way once more, if we use something other than a metal at
low temperatures as intermediate for joining two work pieces we call it gluing. We also have
"sintering" (= heating a compacted powder for some time) as a joining technique
for metals ("powder metallurgy") but that
technology is more prominent with ceramics.
In all these cases the metals to be joined are not
liquefied. |
|
 |
We also may join metals by riveting or by screwing them together.
If you ponder this a minute, you realize that joining metals (and other materials) is at the very heart of making machines
or just about everything more complex than a flint stone tool. |
 |
Back to liquid welding. So first it's liquid,
now it's solid - there is no principal difference in what we now call liquid welding to casting,
the amazingly complex process we just covered. |
|
 |
This means that all the problems
discussed above in the context of casting and general solidification apply just as well to liquid welding.
In particular,
the structure and thus the properties of the solidified parts of the weld seam might
be completely different from the structure in the unaffected metal. Moreover, the structure in the heat
affected zone next to what was liquefied might be different too. After all, that part, though not molten, was
heated up to very high temperatures and cooled down again rather quickly.
Almost anything can happen. If your weld
seam is far softer, far harder, or even brittle compared to your metal, you got a problem. |
 |
It is a big problem. So big that we must accept
that a lot of metals cannot be welded at all - and that includes some steels! |
|
 |
Next time you go places with an airplane, look at it closely. What
you will see is that it's metal parts are riveted together, not welded. Airplane bodies
consist mostly of an aluminum alloy and those are very difficult if not impossible to weld.
Is the body of your car
welded, soldered, glued, riveted or screwed together? Do you know? Yes? Good! Now go
and ask your wife; she probably neither knows nor cares. You know, of course that your car parts are welded together - or
possibly glued? This apparently asinine question is not quite as stupid as is sounds; ask your BMW dealer, for example.
Pretty much all cars (except for some high-end Audis) are made from weldable steel. If you can't weld the body together,
you have a big cost problem. Same thing for ships and plenty of other steel constructions. One of the problems encountered
when big things like ships were welded together for the first time, supplanting time-consuming and expensive riveting, can
be found in this link. |
|
 |
By the way, next time you encounter an old
iron construction like a bridge or a train station, look at it closely, too. The whole thing is riveted together for sure.
People couldn't weld steel before 1920 or so, and all the huge steel construction coming into being in the late 19th century
were riveted together because solidification of steel hasn't been mastered.
Even with modern technology, the weld seam
is the weak point in a steel construction as some pictures in the link, including a horrible one, amply demonstrates.
The picture below, showing a cut through a weld seam, makes clear why. The visible structure is mostly from the cut; the
microstructure would not be visible a this low magnification (maybe 10 fold). Nevertheless, it is clear that there are major
disturbances of the microstructure. If you optimized the microstructure of your material, it can only be worse at the weld. |
|
|
| |
| |
| |
|
 |
Good weld seam
|
| Source: Internet; sorry can't find it again |
|
| |
| |
 |
However, liquid welding is not so interesting
in the context of sword blades. You, the ancient smith, couldn't melt your iron and steel anyway, remember?
So you could neither cast it nor liquid-weld it.
We will not pursue it any more except for pointing out the epiphany we just had
|
| |
|
| |
You cannot simply weld the pieces
of your broken blade together
by using liquid steel.
|
|
| |
|
 |
So liquid welding is out. But weld we must
if we are to make a "pattern welded"
damascene blade or just about any iron or steel object for the
first 3.000 years of iron and steel technology.
How about just banging two pieces of the same material together? For
chewing gum or wax this kind of works. It doesn't work for iron or other metals, however. Try it. Take two nails, two sheets
of aluminium foil, whatever, put the pieces on top of each other and bang away with your hammer.
However hard or cunningly
you hit the pieces, they will not weld together. But you, the smith, knew that anyway. You also know how to do it right:
You must heat your two iron or steel pieces to a rather high temperature before you start your banging. Then the two pieces
might stick together. If you sprinkle some "magic" stuff on the hot surfaces to be welded together, it works even
better.
This is a kind of "fire" welding that pretty much all smiths did it and still do to this very day. |
|
 |
You take two pieces of iron or steel, make them hot, sprinkle some
"sand" on them, and bang them together with your hammer. If properly done, the effect is the same as liquid welding:
two pieces have been joined to form just one piece without any intermediate layer of solder or glue.
|
| |
|
| |
|
| |
|
 |
So how does hammer welding at high temperature
work? Before we look at that, let's consider the reverse question: Why will two pieces of iron brought into close contact
at room temperature not stick together and get "contact-welded" just so? Or
at least after some severe banging?
After all, a metal atom at the surface has some free space around it that it would
love to fill with other metal atoms. We know that it always wants to have a lot of neighbors around: 12 for the fcc and
hex crystal structure and still 8 for the bcc structure (back
to chapter 4.2.2 if this doesn't strike a chord). |
| |
Some heating is still required to provided the energy for the necessary grain
boundary formation. What you get for silicon (Si) is shown below: |
| |
|
| |
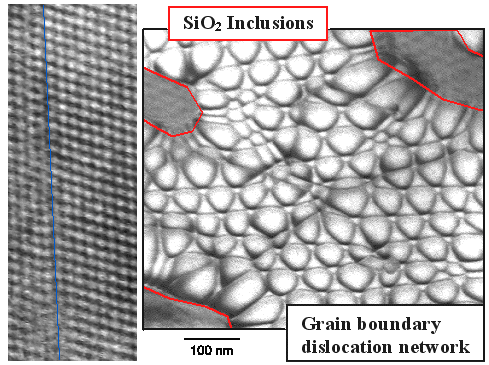 |
| Grain Boundary after contact welding rather perfect silicon. |
Left: Looking at the "seam" edge-on with atomic resolution. The two
crystals have joined rather perfectly in parts.
Right: Looking at the contact welding seam from above (top view). A complex network of dislocation is present, constituting the unavoidable grain boundary. Some oxide inclusions are seen too. More in this link. |
|
| |
|
 |
I tried to align the two wafers with respect to their crystal orientation, but
you can never do it so they have exactly the same orientation. A ("small angle")
grain boundary still is needed, and it is a complex creature as you can see in the picture above.
What, exactly, do
we see in the picture? |
|
 |
The left-hand atomic resolution picture shows a perfect weld seam with some little mismatch
of the crystal lattices. It does not show much else for the reasons given
before.
In the right-hand picture we have the top-view and look at the weld-seam area from above. The complex looking
network is nothing but the required dislocation structure needed for the kind of grain boundary we have here. More interesting
are the shapeless gray blobs, circled in red. They are small silicon oxide (SiO2) particles that got embedded
in the weld seam despite all my efforts to keep the welding nice and clean. They correspond to the slag inclusions incurred
when hammer-welding steel.
Without some heat and pressure one only gets weak welding for the reason given in item 3 above. Nevertheless, the best we can do in contact welding nowadays
is to use ultra-clean, ultra-flat, single-crystalline silicon wafers with the same orientation as far as possible. I show
you the results here just to demonstrate that even for the best possible conditions,
you get a rather huge mess. If you bang the whole package with your hammer, the mess will not get smaller. |
 |
Now let's consider contact welding for steel.
We certainly don't have atomically flat surfaces, the steel at all times is covered with a thin (some nanometers) oxide,
and the crystal orientations don't match because it is a poly crystal anyway. |
|
 |
If you now heat up your steel to red-hot temperatures in air and thus in oxygen, it simply
burns, forming comparatively thick iron oxides called "scale".
Scale can grow rather thick; you can get fractions of a millimeter in minutes!
If you bring scale-covered steel in
close contact, not much will happen. However, if you put your iron in the reducing part
of you fire (deeper inside) in contrast to the oxidizing part (more on top of the flames)
you minimize scaling. If you also pour fine-grained "sand" that contains silicon dioxide (SiO2) on
the hot steel, you may liquefy the scale. If you now hit the two pieces hard with your
hammer, the liquid stuff squirts out at the seams and you get the iron atoms into close contact. Plenty of thermal energy
does the rest. Iron atoms will move and bond to other iron atoms. Welding is achieved.
If you keep your material hot
enough for a while, grains grow. The grain boundaries formed during welding thus move and become unrecognizable from the other ones. Taken all of that together, there are plenty
of reasons why you need to do hammer welding at elevated temperatures. |
|
 |
Of course, hitting hot steel with a hammer also produces a lot of dislocations—but so
what! At high temperatures they are likely to disappear quickly, and the steel structure changes completely anyway as soon
as you go below the austenite-ferrite transformation temperature at about 720 0C (1.328 0F).
However: Any slag particles locked in the seam will remain there. |
 |
So hammer welding works. But if you look at the silicon figure again, and consider
that this was done under far more perfect conditions than contact welding with steel but still has some "dirt"
(silicon dioxide (SiO2) particles) in the weld seam, you must expect that the a seam after hammer welding will
not be overly perfect either. |
|
 |
This is indeed the case. Old pattern-welded blades, for example, are often more
corroded down the hammer-weld seam than in the bulk of the blade. This signifies imperfect seams. If you can see a pattern
in an X-ray image, it signifies that
corrosion was different in different parts because all kinds iron and (carbon steel) look exactly the same in an X-ray image.
Contrast differences then are exclusively due to differences in thickness since slag particles are too small to show. |
| |
| |
| |
 |
| What the blade looks like. Three layers of torsion damascene are visible |
X-ray image. White corresponds to thinner parts. |
| Parts of a Viking blade (8th - 9th century) |
| Source: The wonderful book of Manfred
Sachse |
|
| |
| |
|
 |
You also can see slag particles in hammer-welded seams in a standard metallographic
analysis. Here is one example from Buchwald's first book |
| |
| |
| |
|
| |
| |
|
 |
On top the steel is carbon-rich "regular" steel. On the
bottom the steel contains carbon and major amounts of phosphorous. The arrows indicate
weld seams. They all contain slag inclusions. |
 |
The ancient smiths' weren't doing badly, however. Relative to what I could do
under optimal condition with silicon in 1977, they were even doing fabulously! The few investigations made into ancient
pattern-welded seams show extremely high quality and almost perfect hammer welding in some
cases - and extremely lousy jobs in others.
I will have much more to say about hammer welding in the context of pattern-welded
swords. In particular that the ancient smiths', for all we know, could not possibly
have done it.
Since pretty much all remaining iron and steel artifacts from antiquity prove beyond doubt that these
smiths did hammer weld their iron and steel object, it becomes clear a this point that
we do not know enough! | |
|
| |
| |
| |
© H. Föll (Iron, Steel and Swords script)