|
6.2.2 Solidification and the Art of Casting |
| |
What happens when we go from the
liquid phase to a two phase region?
|
|
 |
That was that pressing question on the back of your mind! Or was it? Why should
this be a pressing question? You know what happens when we go from the liquid phase
to whatever solid phase: the stuff solidifies! |
|
 |
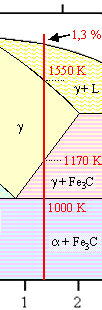
Indeed—but how? If you look at our iron-carbon phase
diagram again and stay right with the 1,3 % carbon composition line we used before, you realize that upon cooling from
the liquid ("L") state you run through several phasefields before you are at room temperature:
- g + L
- g
- g + Fe3C
- a + Fe3C.
The stuff solidifies, alright. But how is that going to happen? |
|
|
| |
 |
To put the question in other words: You and about everybody else too, has a far too naive
view of what is implied in casting something. Ask around and you will find out that
- the askee has never ever given the topic a single thought.
- If pressed, most people would guess that whatever the melt consists of just "freezes".
| |
|
 |
Let's say the melt consists of iron and 1.3 % carbon. Almost everybody out there would expect
that upon "freezing" you get solid iron with 1.3 % carbon "somehow" dissolved in it. Almost nobody would
guess that at room temperature you have rather pure iron with some embedded iron carbide but that at higher temperatures
the solid would be completely different from that. | |
|
 |
Nobody (well, almost nobody) would guess that
the science of casting is a rather complex field.
I grant that you, the ancient
smith, did not cast sword blades or anything else made from pure iron or steel. So why should you worry? Because in order
to understand wootz steel, we need to look at what really
happens when we go from the liquid phase to a two phase region! We will find that the basic difference between wootz steel
blades and all others stems from the fact that wootz steel was liquid once. |
 |
Of course, all we need to do is to learn a few more things about phase diagrams.
Then we give the iron-carbon phase diagram a close look and figure out what happens when we go from the liquid phase to
the solid phases. It should contain that information, after all. It does, indeed, but the iron-carbon phase diagram is also
a rather complex one. You might wonder if most of the other »8.000 binary phase diagrams
are much simpler? Maybe only iron and carbon produce such a complicated mess?
The squiggly lines "»",
by the way, mean simply "about". I'm going to use common science abbreviations more often from now on because
at this point the faint of heart or soft of brain are no longer with me. |
|
 |
So does the iron-carbon phase diagram belong to the more complex
ones?
Unfortunately or luckily, depending on your point of view, this is not
the case. There are plenty of phase diagrams out there that are far more complicated
than the iron-carbon one; take, for example the phase diagrams for copper (Cu) and tin (Sn), producing what we call bronze,
or copper (Cu) and zinc (Zn), producing what we call brass. They are shown in an illustration module. Both phase diagrams plus plenty of others are easily found in the
Internet.
If you start to feel sorry for our students, consider that this will give them jobs. Having a lot of complicated
phase diagrams is actually good luck because it will give us opportunities for engineering
novel and useful materials for a long time to come.
Simple phase diagrams are not only intellectually boring, there
is just not much you can do with those materials. |
 |
Fortunately for you, there are simpler phase diagrams, too, and
they make it easier to get the point across that I want to make now.
Examples for most simple phase diagrams include
the gold - silver, or copper - nickel systems. Here we give a quick look at a slightly more complicated phase diagram: a
mixture of lead (Pb) and tin (Sn), including common solder. |
| |
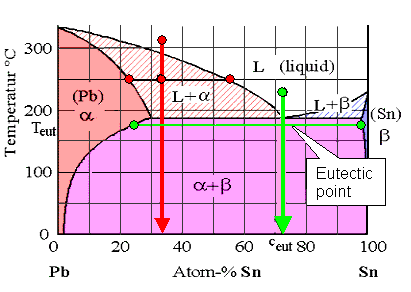 |
The lead-tin (Pb-Sn) phase diagram
Solder is the Pb-Sn composition
at the eutectic point (» 73% Sn) |
|
 |
Let's start with a composition around 73 % tin (Sn) (the green state point in
the figure above). Cool this mixture down and it will solidify directly from the melt
at the "eutectic point" into a two-phase solid. It will not
go through a phase field where it decomposes into a mixture of liquid and solid with different
compositions as we had before. The solid thus has
exactly the same composition as that of the melt. It decomposes into two different phases (a and b in the example above) but
the composition is the same as the melt.
That's what the phase diagram tells you. As you can also see, the eutectic composition that we have at an eutectic point is by
necessity the composition with the lowest possible melting point of
a binary system. |
|
 |
Now you realize why "eutectic
" is such a magic word in working metals! If there is an eutectic composition
in your phase diagram, it makes for easy casting:
- The melting point is as low as it will ever get.
- The composition of your melt will be the composition of your solid - everywhere!
|
|
 |
"Eutectic", by
the way, is from ancient Greek and means: "easily melted". Aha! |
|
 |
To be sure, the solid just below the eutectic point does decompose into tin-rich
lead and almost pure tin as shown in the phase diagram. But the over-all
composition is unchanged.
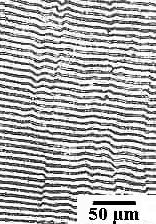
What a typical eutectic microstructure looks like is shown
in the following picture: |
|
|
 |
 |
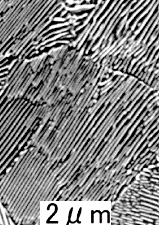 |
| Eutectic microstructures
|
Solder (Sn-Pb)
Lamellae of tin
(with a bit of dissolved lead) and lead (with a bit of dissolved tin) form a "zebra-stripe" pattern |
Al-Cu (33 wt%) eutectic alloy.
Lamellae of aluminum (with a bit of dissolved Cu) and copper
(with a bit of dissolved Al) form a "zebra-stripe" pattern |
Steel.
Eutect
oid composition (I'll get to that).
Lamellae of cementite and ferrite (with a tiny bit of dissolved carbon)
form a "zebra-stripe" pattern |
|
|
 |
Two things are obvious:
- There seems to be a very special "zebra stripe" structure for
eutectic compositions.
- This "zebra stripe" structure seems to occur for all (?) eutectic systems as demonstrated by the three examples,
one of which is eutectoid (?) steel.
I'll get to the question marks in due time. Nevertheless, if you want to understand steel, you must acquire a bit
of familiarity with eutectics and close relatives. |
 |
What you realize by now is that solidification in general and casting
in particular is not as simple as it looks, indeed. | |
|
|
 |
Let's look now at non-eutectic compositions, for example
the red composition at about 33 % tin as shown above and on
the right. The melt, according to the phase diagram, decomposes into some lead-rich solid stuff and a tin-rich liquid as
shown exemplary by the two red state points in the L + a phase field.
By the time when the
last droplet of the remaining tin-rich liquid has solidified, the solid has a very inhomogeneous
over-all composition as shown below.
This is nothing new. When you freeze the binary system H2O - NaCl (also
known as salty water), the first solid that crystallizes from the "melt"
is sweet ice that contains very little salt. The more ice you form, the saltier the water must get. The last bit of water
to solidify then must contain all the salt and that ice then is salty. Your ice, in other words, is not uniform with respect
to its saltiness.
If you don't believe me, look up this illustration
module. | |
|
 |
But back to the Pb - Sn system. The chunks crystallizing first are lead-rich compared to the
over-all composition; the remaining liquid then is enriched in tin.
Now comes the crucial questions |
|
|
|
How should one imagine a mixture
of lead-rich solid and tin-rich melt?
Are solid pieces floating around
in the liquid?
|
|
| |
 |
No, no, no!!! Not at all! Consider:
- First, the solid in this case would be "heavier" than the liquid and sink
"like lead" to the bottom.
- Second
, and more important: solidification or crystallization will always start at the coldest
places, and the coldest places for starters are always the walls of the container or the surface.
- Third: This is obviously true for all mixed
solid - liquid phases with the exception that on occasion the solid pieces could also swim on top. Only if the density of
solid and liquid is identical (very rare) would the solid float in any depth.
|
|
 |
As the temperature goes down, more and more liquid crystallizes and the newly
crystallized parts get increasingly tin-rich. The final result is a lump of lead-tin alloy that is lead-rich on the outside
and tin-rich in the inside relative to its gross composition. Schematically, it looks like this: |
| |
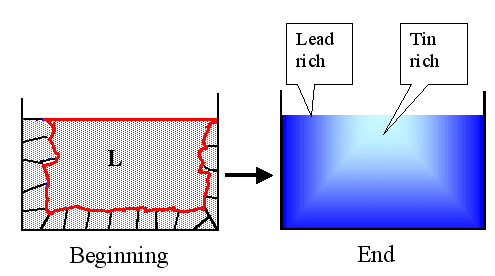 |
Solidification of an alloy and the phenomena of segregation
There will be a lot of grain boundaries (indicated in the left picture)
and dislocations as we have seen before. |
|
 |
We have now encountered the phenomenon of segregation,
meaning that the composition of the solid material is not the same everywhere (as you probably would have liked it to be).
It varies considerably on a "macroscopic" scale, like from the outside to the inside of the crucible or die that
is used for solidification. If we just describe what we get, we say that the components of our mixture got
segregated or separated. And what that means is: |
| |
We have no longer the same composition
everywhere. That means that we have no
longer the same properties everywhere.
This is generally not so good!
|
|
|
 |
Even worse, besides this more "macroscopic" segregation
that leads to variations in the composition of the solid on a large scale, there is also something called "micro segregation" on a small scale. This is shown below for our example from above |
| |
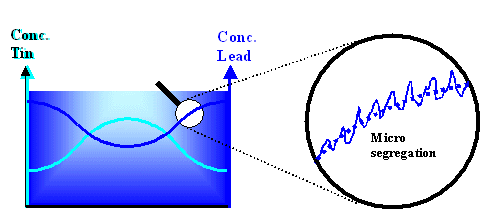 |
| Macro segregation and micro segregation |
|
| |
 |
On the left the variation of the lead and tin concentration due to macro segregation
, coming right from the phase diagram, is shown schematically. On the right we look at just the lead concentration in
a small region (millimeter or smaller) The concentration fluctuates more or less periodically, due to micro
segregation effects, around the average values (dotted line) given by macro segregation. |
 |
Micro segregation results from reasons not contained in the "nirvana"
phase diagram. We will give it a closer look because it is directly responsible for the "water
" structure of wootz steel blades. |
|
 |
So you better get used to the term segregation.
It is is a key phenomenon for sword making (and for most everything else).
In human affairs segregation is usually considered
to be bad. In Materials Science affairs, segregation is neutral.
From the viewpoint of the material,
macro segregation is good because it brings the system closer to nirvana. And everybody is entitled to pursue happiness,
after all.
From the viewpoint of the Materials Engineer, segregation is often bad. However, you couldn't make wootz
blades without it, so for this purpose it is good. It is also great if you want to clean your material. If you want sweet
ice from water contaminated with salt, let if freeze and take only the parts that froze first. They are clean. The same
principle applies of you want extremely clean silicon, and so on. |
|
|
 |
Nothing helps, you need to understand at least the basics of macro- and micro
segregation because we need to manipulate that behavior quite a bit to make sword blades. If we deal with segregation the
right way it will not bother us too much anymore, and we might even use it to our advantage like in wootz steel. I will
give you some hints here, use the science module if you want to learn (a lot) more. |
|
 |
We already know that macro
segregation is a direct consequence of a phase diagram, which in turn is a direct consequence of the second
law.
So let's now give a first quick look at some of those many new
things that might cause micro segregation. |
|
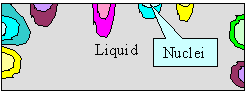 |
 |
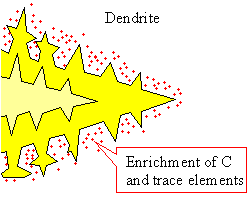 |
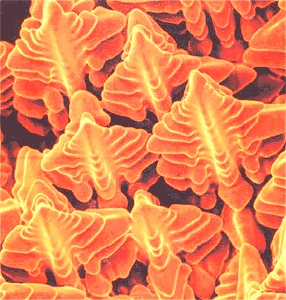
Nucleation of grains and dendritic growth
|
Upper figure:: Schematics of nucleation and
first grain formation.
Lower figure: Better schematics of first dendritic
grain formation; a kind of "enlargement" of the yellow grain above. |
Scanning electron microscope (SEM) picture of dendrites in a nickel-based super alloy. You
get a picture like that by starting solidification and then quickly spilling the melt during the process |
| Source: S.A. David et al. |
|
 |
When I introduced you to grain
boundaries, we looked at solidification at some solid - liquid boundaries advancing into the material from various nucleation
points typically found at the surface of the container. Look at the relevant
figure once more to recall what I mean. Now notice that the way it is drawn is far too simple
with respect to reality.
In the figure above parts of the old figure are shown once more. If we would look a bit closer
at one of the growing crystals like the yellow one, it would often look like the "dendrite" shown above.
|
|
 |
Dendritic or "tree-like" growth
is fairly common whenever the "driving forces" are large (I'll come to that).
For the time being, just note that you have large "driving forces" when you
cool down quickly.
What happens then is fairly complex, if well understood. I'll
give you details later. In essence the solidifying part releases "heat of solidification " and thus heats up itself plus the liquid around it. That makes solidification
more difficult and slows down the advancement of the dendrite tip into the liquid. |
|
 |
Heat of solidification is simply the energy no longer needed if the atoms don't
run around in the liquid anymore. As soon as we have a solid, the atoms are tied down to a place in the crystal lattice
where they can only vibrate so much. We covered that already.
You know that effect quite well, you just didn't give it fancy names. Put some very cold water close to the freezing
point into your freezer. It won't freeze instantly because the energy released as "heat of solidification" heats
it up again. Your freezer must transport this energy to the outside world, and that takes a while.
Same thing in reverse.
Put your frozen soup into a pot and put it on your range going at full blast. It will not liquefy instantly; it actually
takes quite some time. Now you have to supply the heat of solidification or the heat of melting, same thing, and that takes a while. |
|
 |
We already have a first reason why solidification could be a stop-and-go process
even for pure materials. Let's say the solid-liquid phase boundary advances rapidly into the liquid part at some time. Then
a lot of heat of solidification is produced, the temperature goes up and things slow down. Now less heat of solidification
is produced, the temperature goes down, and things speed up once more. And so on. |
 |
If the melt contains any impurities, things get more complicated. Impurities usually
like it better in the liquid (look at the phase diagrams!) so the solid will have a lower concentration at first. A heat
of steel always contains all kind of impurities, so solidifying steel is always rather
complicated from a segregation point of view. |
|
 |
The liquid close to the solid-liquid phase boundary thus will get impurity-richer
because it "get's" all impurities not accepted by the solid. The impurity concentration close to the the solid-liquid
boundary is thus larger than it ought to be. That always means that the local melting
point is now lower than it ought to be. This makes solidification more difficult, it slows down or comes to a stop.
Meanwhile, the surplus impurities in the melt diffuse into the bulk of the melt and their concentration close to the solid-liquid
phase boundary goes down. That means the local melting point goes up, and solidification speeds up.
Once more, good
reasons for a stop-and-go process of solidification or, to put it more precisely, a fluctuating growth speed of the solid. |
 |
To summarize and generalize: There are reasons why the solid-liquid boundary should
not advance with constant speed into the liquid. In other words, there are reasons why the growth speed of the interface should fluctuate or oscillate. There are also reasons why this should
not happen in lock step everywhere but in different ways at different places. |
|
 |
Be that as it may, the decisive parameter in the end is the (local) growth speed,
meaning how fast the solid-liquid interface advances into the liquid. The reason for that is simple: How much impurities
are incorporated into the solid depends on that growth speed. |
 |
You should protest here and tell me that how much of an impurity end up in a solid
depends on the phase diagram and nothing else! It should thus not depend on the growth speed of the interface! |
|
 |
You are right! And I'm right, too. We both can be right about a mutually exclusive
item because, as I stated long before, "Phase diagrams show only
the "nirvana" state". A freshly solidified piece of material may not be in that desirable state, however! |
|
 |
Looking at this a bit more closely (with equations), it turns out that the phases
predicted in the phase diagram will only come into their ideal being if the solid-liquid boundary advances v e r y s
l o w l y. If it advances with normal speed, the expelled impurity atoms close the solid-liquid boundary do not have enough
time to move away from the boundary into the depth of the liquid. What then solidifies next is a liquid different
from that in the phase diagram. You will now incorporate more impurities in the growing crystal. |
|
 |
That's sounds more complicated than it really is. All it means is that the fraction
of impurities ending up in the crystal relative to those in the melt goes up with increasing growth rate. Keeping this in
mind, let's see what happens now if we solidify a melt containing two kinds of atoms. |
 |
Let's assume that we have some fast crystallization at the solid-liquid interface
of some growing grain. We have already seen that several things conspire to cause the growth rate to fluctuate or oscillate, meaning that crystallization happens in a stop-and-go fashion. Let's look at this
again but now also keeping in mind the impurity incorporation into the crystal: |
|
 |
- During fast
crystallization many of the impurities contained in the melt will end up in the crystallized part because there is
just not enough time to keep them all in the melt or to expel them from the solidified crystal. Those parts of the crystal
that solidified fast therefore will have a relatively high impurity concentration; above of what the phase diagram predicts.
However, while crystallizing fast, a lot of
heat of crystallization is released that heats up the melt next to the solid-liquid boundary to a temperature possibly above the melting point. Crystallization has no choice but to stop or at least to slow down.
- During slow
crystallization most of the impurities remain in the melt, enriching it locally beyond the concentration of the basic
composition. That means that locally the melting point now comes down, slowing down
crystallization even more.
- Now the impurity atoms in the impurity-rich part of the melt have enough time to move away into the depth of the melt;
their local concentration goes down. That means your local melting point goes up while
the general temperature still goes down because you keep cooling your system. Crystallization speeds up and becomes fast again.
- Back to 1.
|
 |
OK - I admit that the phenomenon of constitutional
supercooling is a bit hard to understand when first encountered. It is even hard to calculate
if we look at it in three dimensions and not just one-dimensionally as implicitly done in the prose above.
In the final
result, however, it is quite likely that the sold-liquid interface moves in a stop-and-go fashion in not just one but in
all three dimensions. This produces not only the characteristic dendritic shapes but
also a more or less periodic variation of the impurity concentration in the solid. |
|
 |
Here is the reason for all kinds of what we call "pattern
formation" in solid materials, and that includes the patterns found in wootz steel. We like
that pattern and see it as aesthetically pleasing and a sign of quality.
It also includes pattern we find in the ultra-pure
silicon (Si) crystals we use for making chips. We hate those patterns but must live
with them. More about that in the science link.
Segregation is rather the rule and not the exception when you solidify
liquids. We will look at this some more in later chapters. | |
|
|
 |
Pattern formation by segregation effects comes with many names. They may be called
"banding", or "striation"
and always involve a non-uniform distribution of impurities. Of course, the effects of segregation have been observed and
described centuries before they were understood. |
 |
Sorry. I now destroyed your naive view that solidification and casting is a simple process. Just pure some liquid into the mold, wait a while, and take out the solid product?
Ha! |
| |
|
© H. Föll (Iron, Steel and Swords script)