Phase Diagram of Salt Water
You should now be able to draw some conclusion on your own.
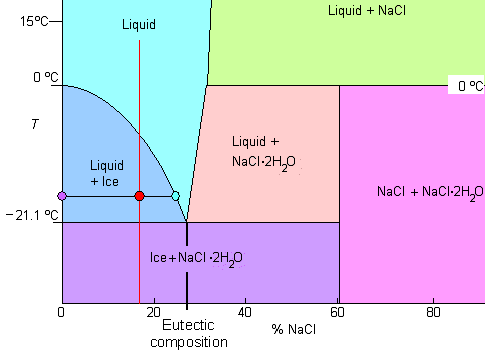
|
| Phase diagram of salt water. |
Never mind the NaCl end! NaCl · 2 H2O means that you have a rock salt crystal with water molecules as a kind of dissolved impurity (2 H2O molecules occupying the place of one NaCl unit). The key word in this context is "crystal water" - look it up!
- There is an eutectic composition around 27 wt % NaCl or salt dissolved in the water.
- At the eutectic point, the melting or freezing temperature is as low as it will get: -21.1 oC (- 6oF)
- The maximum amount of salt you can dissolve in water is around 30 %, slightly increasing with temperature.
- If your salt concentration is, for example, lower than the eutectic concentration (i.e. it is below about 27 %), the system will decompose into salt-free ice and liquid water with an increased salt concentration as shown for the "red" composition around 18 % salt
- The last drop to solidify will do so at -21.1 oC (- 6oF) and contains about 27 % salt in liquid and ice.