| |
|
 |
Around the year 2000 I wrote my first short module about ancient iron and steel
as part of one of my "academic" hyperscripts. In those ancient times there was practially nothing in the Internet
about that topic (there wasn't even an Internet a few years before that). Googling for "steel history" invariably
put my module on the first place in the following years. Checking right now (Nov. 2013), it actually still makes second
place. |
|
 |
But now you have plenty of other entries, including sites of many professional
and amateur smiths who make wonderful things by emulating old technologies. Moreover, lots of people (mostly male) have
fun on weekends by throwing together a bloomery, trying to make some iron / steel. Either just as a fun thing to do (often,
it seems, with the secondary goal to prove that you need quantities
of beer for making iron) or as project for youth groups, school classes, or other more or less organized hordes.
Some go for one try and typically do not produce much iron or a nice bloom but have fun anyway. Some, however, get ambitious,
keep going, and develop tremendous "hands on" experience, producing large and rather good blooms.
Search
the Net yourself and you will find plenty.
In what follows I just show some blooms and schematic drawings of bloomeries
to illustrate a few points made in the backbone. |
| |
| |
| Blooms
(German: Luppe) |
 |
The first picture shows modern blooms. The top two pictures show
what you get if you let your bloom cool to room temperature without "working" it (have it "wrought")
while still hot. You wouldn't know that there is a lot of iron in these ugly bastards.
The lower two blooms are still
hot, and you can get an idea why in English this lump of metal might be called bloom = blossom of a plant. In reality, however,
it is far more likely that the name goes back to the Old English "bloma" = "lump of metal". |
| | |
|
|
|
 |
| Iron blooms |
| Sources (clockwise): Photographed in a museum (Reisberg), Mineralien Atlas, Project
of the Graf Engelbert Schule, Bochum, The Little Princess Bloomery. |
|
| | |
|
|
 |
The
bloom in the lower right picture was split while still hot because it was too large for banging it up with a human-wielded
hammer. This is a very professional bloom, made by Lee Sauder and Skip Williams in their Little Princess / Rockbridge bloomery.
Little wonder! It resulted from experiment No. 84; those guys are experts by now.
Smelting datas were: Charge Interval
10 minutes, total ore weight 45 lbs = 20.4 kg, duration 4 hours 20 minutes from first ore charge to bloom removal. Bloom
weight 18 lbs = 8 kg. Assuming that the ore "richness"
was around 60 %, this amounts to an efficiency around 65 % - which is very good! |
 |
Next we see a slightly rusty bloom, probably not condensed by hammering, that
was cut open to reveal the internal structure. Big lumps of charcoal are stuck inside. |
| | |
|
|
|
 |
| Cut open bloom |
| Source: "Alphabet der Heimatkunde" for the Town of Stolberg, Germany and V.L.
Thelen, Mies van der Rohe Schule, Aachen, Berufskolleg für Technik. |
|
| | |
|
|
 |
The sectioned blooms below show the "spongy" nature of all blooms. The same question
as for swiss cheese comes up: How do the holes get into the cheese / bloom? For swiss cheese we know (Look up "Ostwald ripening"), for blooms I'm not so sure. The holes might have been
made by charcoal now burnt off or fallen out, by slag, or by ??? |
| | |
|
|
|
 |
| Sponginess of blooms |
| Source: Internet at large. |
|
| | |
|
 |
Here are some old blooms. Not all that old - Charlemagne
wielded a magical sword around this time in Europe. The interesting parts about these blooms is that they are reported to
contain 2 % - 4 % carbon. I'm not sure that I believe that - at least the 4 % bloom would have melted. |
| | |
|
|
|
 | | Blooms from Yokodaido, around 800 AD |
| Source: Jap. Archaeological Associaton (archeology.jp) |
|
| | |
|
| |
Bloomeries
(German: Rennofen) |
 |
Bloomeries from the outside look rather unassuming, see below. Watching a working
one from the inside would be far more interesting but for obvious reasons nobody has managed to do that yet. That's why
we make "schematic drawings" that always involve some guessing and uncertainities. |
| | |
|
|
|
 |
| Source: Internet at large |
|
| | |
|
 |
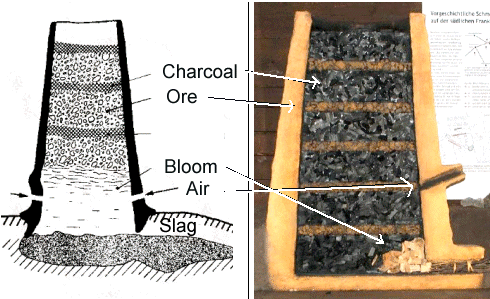
Here are two quite similar "schematics". They can serve nicely to addres
a few problems found with most of those schematic drawings. Can you see some? |
| | |
|
|
|
 |
| Bloomeries, schematic (and with problems) |
| Source: Found in museums and brochures about towns / areas |
|
| | |
|
| |
 |
Here is a list of questionable stuff:
- The burden is layered. That may well be the case in some smeltings but you may also feed a continous mix of charcoals
and ore. Even if there are layers, we don't know if that was done for convenience or out of necessity. If having or not
having layers makes no difference, it is simply easier to pitch a basket of ore into the smelter top, then after some time
three baskets of charcoal, after some time a basket of ore, .... Saves mixing the stuff. But maybe it is important to have
layers? Personally, I don't know - but I have theorized about that.
- The ratio of ore to charcoal is reversed. On the left we have more ore than charcoal, on the right it is the other way around.
- Even in the picture on the right (the more realistic one) the ratio is wrong. The density
of ore is about 20 times larger than that of charcoal. Providing for a ratio
of charcoal to ore of 1 : 1 by weight then implies a ratio of about 20 : 1 in layer
thickness.
- The mechanics of the burden is often quite unclear. It weighs something, after all, and must sit on something else if
it is not supposed to just fall down. In the left picture this is unclear. If the slag is liquid, the whole stack then must
swim on top? In the right picture this problem does not exist since it is a picture
of a model where the real charcoal etc., by necessity, sits on the floor. But the model is lacking the slag!
- One or two tuyeres? Does the tuyere have to extend somewhat into the inside? Or is it just a hole in the wall?
|
|
 |
I'm not trying to put blame on somebody. Whowever made these picture was aware of the fact
that you just can't get everything right in schematic drawings. We had the same formalistic
problem with schematic drawings of crystals. The real
problem is that people looking at the pictures are tempted to see it as the real thing. |
 |
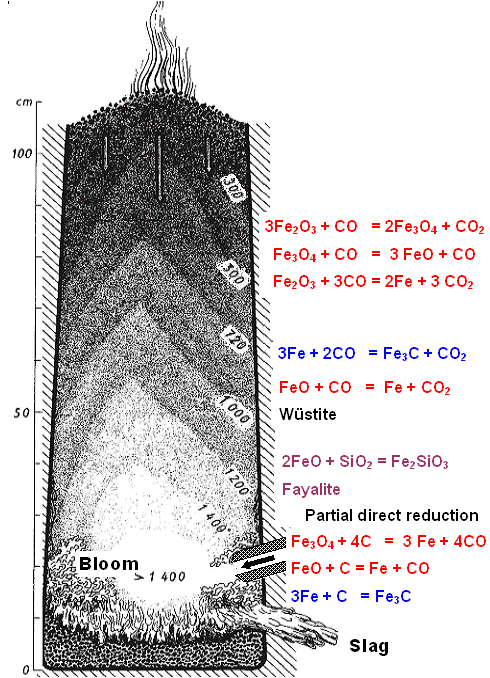
Here is a beauty; it illustrates my
"theoretical" concept of having the part close to the stack walls much cooler than the inside. It was conceived
after something R. Pleiner, a very well-known archeometallurgist,
must have published.
Notice that ore and charcoal are not layered. |
| | |
|
|
|
 |
| Schematic bloomery after Pleiner (?) |
| Source: Landesmuseum for Vorgeschichte und Denkmalspflege Sachsen Anhalt, Germany: Fund
des Monats, Mai 2008. |
|
| | |
|
|
 |
Iron particles coming down on the outside stay much cooler then the ones in the middle and
thus might make it down without becoming re-oxidized to form blooms below and opposite the tuyere (or all around the wall?).
It is a nice touch that the temperature (in oC) and the various chemical reactions for producing iron,
cementite and slag are shown. However, the reactions for turning
iron back into an oxide are missing. They would take place in the white "1400 (oC)" zone at the tuyere
mouth.
What keeps the burden from falling down, and what we have on the very bottom of the slag pit is a bit unclear,
though. |
 |
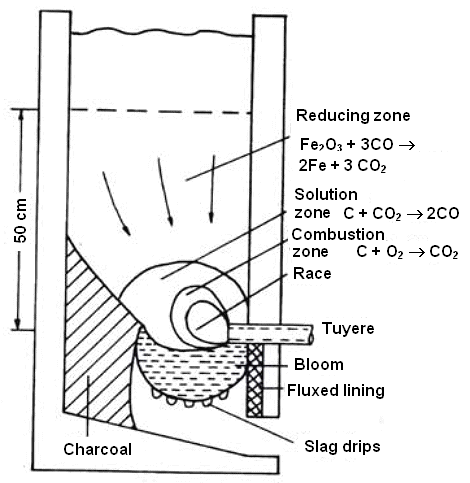
Here is a "Tylecote
bloomery", after the great old man of experimental iron archeometallurgy. |
| | |
|
|
|
 |
| Tylcote bloomery |
|
| | |
|
|
 |
Once more the mechanics of keeping the burden from falling down are not so clear. As a new
feature we have some unreacted charcoal at the far side of the tuyere, indicating that opposite the tuyere it is much colder
than in the middle. |
 |
Finally a more "modern" bloomery made from masonry stones and thus obviously
not given to full destruction after every run. It has several tuyeres. |
| | |
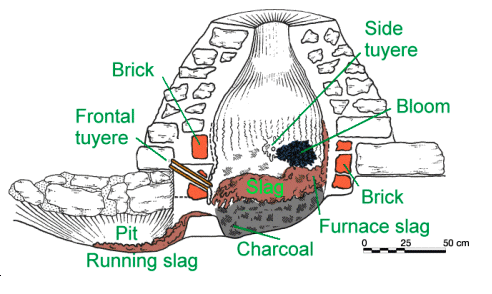 |
| Permanent bloomery |
| Source: Archäologie Baselland; Swiss |
|
| | |
|
|
 |
For unclear reasons it smelts very asymmetrically. The bloom is on the opposite side of the
(main) tuyere and rather high up. Several types of slag exist. Running (= rennen, therefore Rennofen)
slag and furnace slag, obviously the more viscous kind that doesn't run out. Then there are unreacted charcoals at the bottom. |
 |
One might deduce one thing from all these variations and from the results of all
those enthusiasts out there doing smelting experiments: What is going on inside a bloomery is very complex if looked at
in some details. But as long as you do not stray too far from some simple rules, chances are good that your smelter will
produce some iron even if you don't know a thing about the details. The efficiency of production might be lousy, and the
quality of your iron might be abominable, but iron you will get. |
|
 |
It's almost like making a baby. Follow a few rules (mostly for finding a willing mate) and
chances are that it will work - even if the two of you don't have the faintest idea of what exactly is going on if you look
at details.
A major difference between iron and baby making is that a lot of beer doesn't help all that much for the
latter task (just believe me here). |
|
 |
Sorry - I almost forgot: What keeps the burden up there? Nothing much in a bloomery.
It just hangs in there as gravity demands. That's why you have charcoal in the bottom, possibly submerged in liquid slag
if there is enough weight from the top (otherwise charcoal would swim on slag like styrofoam on water). In the huge modern
blast furnaces we can't have that. That's why the have a specific shape with friction keeping the burden in place; look
it up here. We also need to use coke instead of charcoal;
the latter could simply not take the weight without crumbling. |
| | |
|
© H. Föll (Iron, Steel and Swords script)








