|
8. Tuning Carbon Steel |
| 8.1 Keeping Dislocations at Bay
|
|
8.1.1 Being a Drag |
 |
There is an army standing along your eastern frontier. It's a mighty foe along
a long line, with thousands of tanks, heavy artillery, and plenty of soldiers.
You have a far smaller number of lightly
equipped soldiers and not sufficient heavy stuff. You know that you cannot stop the enemy when he advances; all you can
do is to slow him down. You don't even want to stop him because in that case nuclear
weapons would come into the play, completely breaking and destroying the country. How are you going to deploy your forces? |
|
 |
This was the rather real situation that the Germans faced during the cold war.
They weren't supposed to do the impossible and stop the Russians, all they had to do was slow them down (and die in the
attempt) so NATO had enough time to built up invincible forces along the Rhine, keeping those evil Russians out of France.
Stopping them was deemed not to be a good option because that would only have been possible with nuclear weapons, completely
destroying all of Germany for sure. |
 |
There is a long dislocation
on the left side of a crystal, ready to move to the right, deforming the crystal in the process. You only have some foreign
atoms that you can deploy in order to slow it down. Stopping the dislocation is not
a good option because the result would be fracture, destroying the crystal. |
|
 |
What are you going to do in either case? |
| |
|
You could deploy your soldiers one
by one, spreading them randomly and uniformly across the terrain.
They can move around freely (including
running away) and they might just slow down the advancing line of enemy forces somewhat.
If your soldiers are well trained
and motivated the effect might be quite noticeable. | |
You could deploy your your foreign atoms one
by one, spreading them randomly and uniformly across the crystal.
They can move around freely (including
running away) and they might just slow down the advancing dislocation line somewhat.
If your foreign atoms distort the
lattice quite a bit, the effect might be quite noticeable. |
 |
It's a strategy hat would give some results. Nevertheless, it is probably
not your best strategy. So let's look for alternatives. |
| |
 |
You can also get all your soldiers into one place, where they
build an invincible fortress.
The advancing line of the enemy then will go around
your fort, leaving a ring of soldiers behind to keep you under cover. |
|
You can precipitate all your impurity atoms into one big precipitate
that the dislocation can never cross.
The advancing dislocation line then will go around your big precipitate, leaving
a dislocation loop behind to keep you under cover. |
 |
That is obviously not the best strategy either. It is now quite clear
what you have to do. |
| |
 |
Form many small groups of
soldiers and dig them in. That will really slow down the enemy as he has to take them one by one—and that's not going
to be easy. | |
Form many immobile small precipitates.
That will really slow down the dislocation since it has to overcome each precipitate one by one—and that's not going
to be easy. |
 |
Of course, if you don't live in flatland as shown in the figure
below but in a mountainous region, a major insurmountable mountain also hinders the enemy's progress. In crystal terms that
means a second phase (or a really huge precipitate)
that didn't form by getting single atoms together but was always there.
Steel, being a complex material, knows all these
strategies and more, as we shall see: |
|
| |
| |
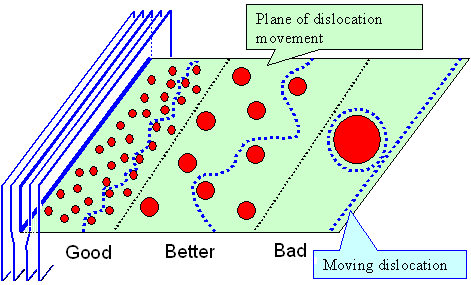 |
| How to obstruct dislocation movement with limited forces |
|
| |
|
 |
If you are a steel crystal, supposed not to yield too easily to outside forces,
the enemy are the dislocations. They start to move as soon as the mechanical stress pressing on your crystal reaches the
yield stress, which is also a measure of hardness.
We will call that "yielding" from now on. Yielding means that plastic deformation
commences. |
|
| |
| |
Remember:
Hardness measures essentially how
difficult it is to move dislocations
|
|
| |
|
 |
Let's consider some steel, some aluminum, or just any
metal with some kind of foreign atoms or impurities inside. Carbon in the case of steel, copper in the case of aluminum,
and so on. We now want to harden the metal, making it more difficult—but not impossible!—
for the enemy dislocations to move through.
We will use those foreigners for that, either as mercenaries fighting single
and on their own, or by forming strongholds involving also some of the good citizens. In other words: by forming metal-impurity precipitates.
|
|
 |
We have names for those hardening mechanisms: |
 |
Achieving maximum hardness without blocking the movement of dislocations completely
(that would make the steel brittle) means to precipitate the carbon, or whatever impurities there are, in an optimized way.
Size, shape and the distribution in the grains of the crystal should also be "just
right". We have two primary tasks now. |
| |
- Finding out what the optimal size of precipitates would be, and
- Forcing the crystal to make the right kind of precipitates.
|
|
 |
After we did that, we need to coordinate solid solution and precipitation hardening
with all the other hardening mechanisms yet to be discussed. This is not going to be easy because you can be sure about
one thing: whatever the right size of precipitates will be for you, the crystal isn't going to like it.
It is now easy
to see why. Get the relevant phase diagram for the impurity you are considering, look at the phase or the phase mixture
you will eventually have at room temperature for the composition chosen, and you will realize that there are only three options: - The crystal wants its impurities dissolved.
- The crystal wants its impurities precipitated.
- A mix of 1 and 2.
|
|
 |
That is true for any binary phase diagram,
not just for carbon in iron.
If the "host" materials wants precipitates like Fe3C in the case of
iron and carbon, or CuAl2 in the case of Aluminum and copper, it wants them to be as big
as possible. I have stated that before a few
times.
We know that by locking unwanted atoms into a precipitate, the crystal gains energy and gets closer to nirvana.
But why are a few big ones better than a lot of small ones? |
| |
|
| |
Why should precipitates be as big
as possible for nirvana?
|
|
|
| |
|
 |
We need to look at this question now in more detail. Especially because the answer
will also tell us why it is so difficult to nucleate a new phase
or precipitate. |
|
There is no such thing as
a free lunch for crystals either. Locking your unwanted impurity atoms into a precipitate does make life better as soon
as your state point hits a two-phase region, indeed. But there is a price to pay. |
|
 |
A precipitate is a three-dimensional defect—remember?
The interface between the precipitate and the crystal is then by necessity a phase
boundary, a two-dimensional defect. You may perceive the phase boundary as a kind of fence that is necessary to keep
the foreign atoms inside.
But phase boundaries are defects too, and thus not something you want. Nirvana is without
defects or at least with as few as possible.
Considering that you must have phase boundaries around your precipitates,
you want to keep their total area as small as possible because their total eneryg scales
right with their area. | |
|
|
 |
In other words, you want the relation between the number of impurity atoms imprisoned
in the precipitate and the the phase boundary "fence" surrounding your "prisons" to be as large as possible.
You want a small fence to lock in a lot of prisoners—and that means you want your jail precipitate
as large as possible. |
|
 |
That's easy to see. It takes far less fence length (one dimensional
thing) to keep hundred prisoners in one large-area jail (two dimensional thing) compared to keeping 25 prisoners each in
4 smaller jails, see below. In three dimensions there is even more to gain by making your prison big. |
| |
|
| |
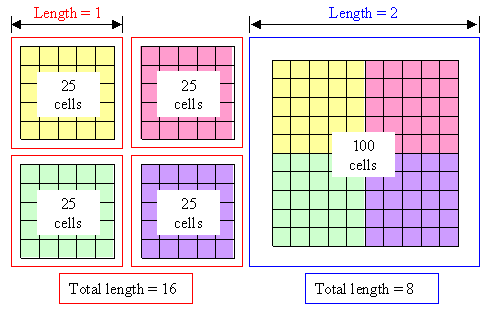 | | Ratio of size and boundary; prison example |
|
| |
| |
|
 |
Four small prisons need twice as much fence than one large prison with the same
capacity. In three dimensions it would be even worse. |
 |
The consequence is simple: If you, the ancient or modern smith, want lots of small
precipitates, you have to fight the crystal and the second law! It's time to review our arsenal for doing this. |
| |
|
© H. Föll (Iron, Steel and Swords script)

