|
The Aluminum (Al) - Copper (Cu) System |
| |
What is It? |
 |
Aluminum - copper alloys are just one member of the large aluminum alloy family.
Or should that be aluminium - alloy? You may not be aware of the fact that different versions
of the name for the chemical element 13 with the symbol Al
exist: It's aluminum in the USA and aluminium in the rest of the world. Why? |
|
 |
Anyway, aluminum is probably the second most important metal after iron / steel.
It comes in many modifications; here is a very superficial survey: |
| | |
| |
| Major Aluminum Alloys |
| Alloy / Metal |
Typ. Comp. (wt%) |
Properties / Typical Uses |
Pure Al
1000 series |
> 99 Al |
Weak, very ductile, good conductor.
Power transmission lines, "aluminum foil",
capacitor electrode. | Cu alloyed
2000 series |
4% Cu
(+ Mg, Si, Mn, ...) |
Strong, age-hardening.
Aircraft skin, spars, forging, rivets. |
Mn alloys
3000 series |
1% Mn |
Moderate strength, ductile, excellent corrosion resistance.
Roofing sheet, cooking pans. |
Mg alloys
5000 series |
3 % Mg + 0.5 Mn |
Strong, work-hardening, weldable.
Pressur vessel, ship parts, beverage cans. |
Mg+Si alloys
6000 series |
0.5% Mg, 0.5% Si |
Moderate strength, age-hardening, good for extrusion and anodisation.
Window
frames. | Zn+Mn alloys
7000 series |
6% Zn + Mg
(+Cu, Mg, ..) |
Strong, age hardening,
aircraft forging, spars, railway carriage. |
| Li | 3% Li |
Good strength, low density.
Aircraft skins, spars |
| Source: M.
Ahsby's and D. Jones' invaluable book |
|
| |
|
|
 |
Age hardening is just another name for precipitation hardening, work hardening
is just another name for strain hardening. |
 |
The case study in the backbone
thus covers only a tiny bit of Al alloy science and technology. But its insights can be generalized to some extent to all
alloys where age hardening is possible and important. |
|
 |
What I'm going to do here is to dig a bit deeper into the matter, showing how the theory behind
what is happening is extremely helpful and saves a lot of work.
Most everything is based on the source given above. |
| |
| |
| |
Formation of the precipitates |
 |
First, we quench the Al-4%Cu alloy from a solid solution to room temperature sufficiently
fast to to freeze in the 4 % solid solution. We will see below how "sufficiently fast" can be quantified
by theory. |
|
 |
As a consequence, we have a large supersaturation of Cu at room temperature. The equilibrium
mixture, according to the phase diagram, would be 93 % a
+ 7 % CuAl2, and the a phase contains at most 0.1 % Cu. The quenched solution
thus contains 40 times more dissolved Cu than required for equilibrium.
There is thus a large driving force for precipitation,
and nucleation starts quickly, homogeneously, and in many places.
The very first steps must be to get 2,3, 4, ... Cu atoms together in about the same place. Since you cannot produce a proper
CuAl2 precipitate with just a few Cu atoms, the first precipitate formed under the circumstances is something
special, called a Gunnier-Preston zone, always abbreviated as GP zone. This is what it looks like: |
| |
| |
| |
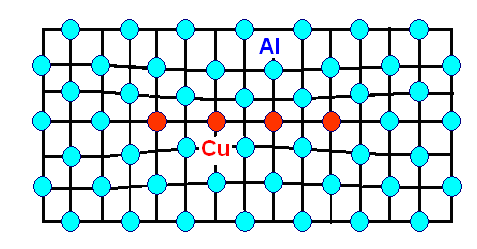 |
| A GP zone is a small disc of the precipitating atoms |
|
| |
| |
|
 |
We substituted some Al atoms in a small disc (max. diameters around 10 nm or so) by Cu atoms.
The lattice then is somewhat distorted as shown, but the GP zone or precipitate is what we call fully coherent with the lattice, i.e. meshing perfectly at the "seams" or the interface. The coherency strain, needed to keep the fit to the Al lattice, is what obstructs the dislocation
movement.
The formation of GP zones simply happens when Cu atoms meet accidentally while diffusing around. They settle
down and become immobile as soon as there are enough (2 might already be sufficient) and form a disc as lowest energy configuration
by slight rearrangements of some of the atoms.
On a larger scale the arrangement it looks like this: |
| |
| |
| |
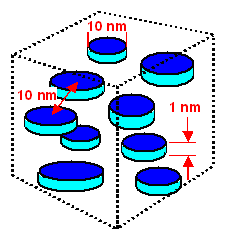 |
Distribution and size for GP zones forming first during the annealing of the quenched Al 4% Cu alloy
Note the scales. They are approximate, of course. |
|
| |
| |
 |
Growth is only possible in one dimension. The strain, and thus also the strain
energy, increase with the circumference or square root of the diameter. This limits growth and thus also the removal of
supersaturated Cu.
Eventually some of the GP zones will also grow perpendicular
to the disc, and a more three-dimensional precipitate is formed. It is not yet proper CuAl2 or the Q
phase but a compromise between the need to "be" Q and to still be coherent and
to fit it into the lattice. Otherwise a large prize would have to be paid in terms of interface energy since the surface
to volume ratio is bad for small precipitates. |
|
 |
That's why these early and small precipitates are called Q''
precipitates. They catch migrant Cu atoms more efficiently than the GP zones, which thus will shrink and release their Cu
atoms which now feed the growing ones.
That's what the Q'' precipitates look like: |
| |
|
|
|
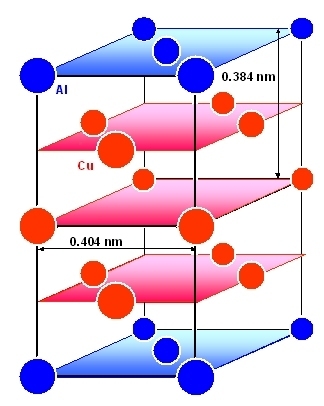 |
Structure of the still fully coherent Q'' precipitate.
The lattice constant of 0.384 nm is a bit smaller than the 0.404 nm for Al, producing some coherency strain in this direction. |
The distribution on a larger scale would look more or less as the one above - except that
you need to add a "0" to every dimension; i.e. 100 nm instead of 10 nm.
Since the thickness is larger than
that of a GP zone, the coherency strain is larger, too. |
|
| |
| |
|
 |
In a way, you just add more Cu planes keeping the basic fcc structure. However, with increasing
thickness the coherency stress and thus also the strain energy increases, and that is rather unwelcome. This limits the
growth of the Q'' precipitates. |
 |
While the Q'' precipitates form and grow, we
still have some supersaturation of Cu albeit much reduced compared to the starting value.
Bear in mind that during
the formation of GP zones and Q'' precipitates there is still some nucleation going on
- all processes always occur in parallel, just with different rate. Heterogeneous nucleation at defects, especially dislocations,
is now the favored mode. You just have to get far more Cu atoms together in comparison to what is described above. That
is more cumbersome and takes longer, but eventually it will win. |
|
 |
These precipitates start out three-dimensionally right away - but are still not yet the proper
Q phase. That's why we call them Q' precipitate. Here
is their structure: |
|
| |
| |
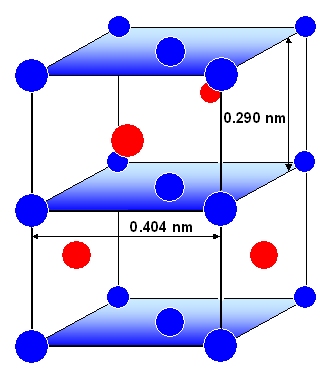 |
Structure of the still fully coherent Q' precipitate.
Some coherency strain is produced but partially compensated by the strain field of the nucleating dislocation. |
|
| |
| |
|
 |
The distribution on a larger scale now looks like this: |
| |
|
|
|
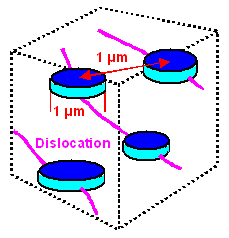 |
The approximate distribution of Q' precipitates, always with
a dislocation at the core. |
|
| |
| |
 |
And we aren't done yet! As time goes on, the final CuAl2 or Q precipitate will nucleate at grain boundaries and at the Q'
precipitates. These precipitates are fully three-dimensional and incoherent. There is
no match of the lattices at the interface, in other words. That makes the interface energy much larger in comparison to
the coherent precipitates but so what! As the precipitates grow to larger and large sizes, the interface energy increases
more slowly with size r (with the square of r) than the
energy associated with coherency strain (increases with the cube of r).
Coherent precipitates thus carry the day as long as they are small but loose against incoherent ones at large sizes.
I won't give a picture Q precipitates because I'm getting tired of drawing all these crystal
models. It's also a more complex structure, not so easy to draw. |
|
 |
So the Q precipitates always win in the end. The Q' precipitates will duly shrink and disappear, and eventually only Q
precipitates are left behind, which then commence to increase in size while decreasing in density by regular Ostwald ripening. |
 |
Why oh why? Easy. Making proper Q precipitates
right away is too difficult. Or, in other words, the nucleation takes a lot of energy and time. So the crystal does what
is easiest to start, the "realizes" that it can't finish this way and switches to then next process. Some people,
I hear, have switched to different life styles and spouses (repeatedly) for essentially the same reason. |
|
 |
You can compare that to two guys doing a long distance race. The one who starts out very fast
but gets slower and slower as time drags on will eventually be overtaken by the one who starts slower but keeps his speed
constant.
You did similar things yourself. You didn't learn to read and write fluently right away, you started with
spelling and writing out every letter by itself. It's easier but it won't get you very far. Eventually you switch to the
more difficult to nucleate learn cursive script, and you stop to spell out words because you learned to
recognize whole words at a glance. Same thing with playing the piano or another demanding musical instrument. You typically
start with a recorder, getting quick results. It just won't get you very far. Then you start again with a more complex instrument,
and playing the recorder fades into the background. |
|
 |
Seen in another way, it's all about probabilities. In the beginning it's just more likely
that a few Cu atoms stick together in disc form to make a GP zones than a number of them in a complicated arrangement for
a Q precipitate. |
|
| |
| |
Understanding what Determines Hardness |
 |
We have seen that during tempering several kinds of precipitates form while the
concentration of dissolved Cu atoms goes down. Solute atoms and precipitates influence dislocation movement in some ways
that depends on their nature, size and density. |
|
 |
We can actually calculate most of that. The result can be drawn into the hardness-time
curve given before. Somewhat simplified, it looks like this |
| | |
|
|
|
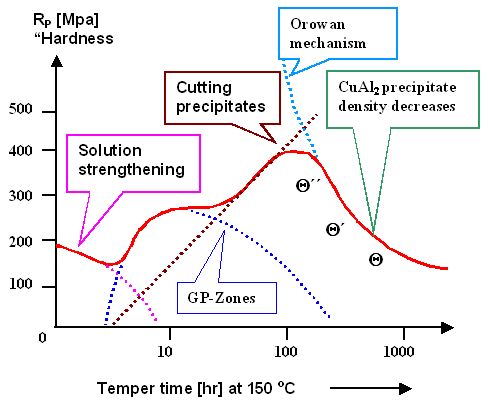 |
| Hardening a Aluminum - 4% Copper alloy |
|
| |
| |
 |
Let's look at what is happening bit by bit. |
|
 |
At the beginning of time there is only
solution hardening. This gives the red curve followed by the red dots,
and hardness decreases because the concentration of dissolved copper atoms goes down. Solution hardening is well understood,
the general relation between hardness or (better) the critical shear stress tsol
needed to move dislocations and the concentration of the solute atoms csol is |
| |
|
| | |
|
| |
| |
| |
and ksol is some constant describing the effect of the particular solute atom chosen.
|
|
 |
The concentration of of the dissolved Cu atoms goes down because GP zones are formed. The
way they influence dislocation movement is not much different from that of single solute atoms. At the beginning we only
have a few small ones that are less effective than the remaining dissolved Cu atoms. But as times goes on, more and more
GP zones are nucleated, their density increases, and their influence on hardness is felt.
The influence of the GP zones
on hardness is shown by the blue dotted line. After reaching a maximum, the curve goes down again because we now form Q'' precipitates, |
|
 |
Precipitation hardening is well understood.
In essence, dislocations can deal with precipitates in two ways. Small and coherent precipitates (including Q''
precipitates) are simply "cut" like this: |
| | |
|
|
|
 |
| A dislocation running through a small precipitate, "cutting" it. |
|
| |
|
|
|
The stress needed for cutting increases with the size of the precipitate. If "cutting"
would be the only mechanism, the hardness would go up about linearly with time since the precipitates get bigger and bigger.
This is shown by the dotted black line above. |
|
 |
However, for big precipitates the second mechanism called
"Orowan
mechanism" becomes operative as shown below. The effect of "big" precipitates
does not depend on its size, only the average distance <l> between the precipitates is important. It
is related to the density rprec via <l> µ
(rprec)1/3.
We have |
| |
|
|
|
|
| |
|
|
|
and G is the shear
modulus, b the Burgers vector
of the dislocation in Al.
The way the Orowan mechanism works is shown here: |
| |
|
|
|
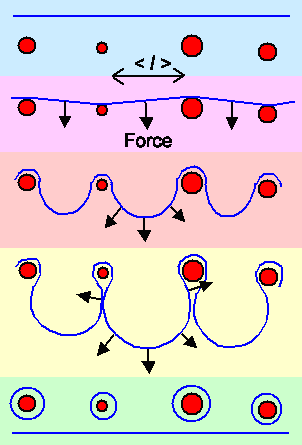 | A dislocation approaches a bunch of precipitates. |
| The dislocation is arrested at the precipitates |
| In between the precipitates the dislocation bows out. The arrows show the forces acting on
the dislocation line. | | The bowed out parts are about to touch. |
| Dislocation loops are formed around the precipitates and the dislocation moves on. |
| The Orowan mechanism. |
|
| |
| |
|
 |
The stress to produce small "bows" is larger than for large ones. It's like blowing
up a balloon. It takes more effort to make its radius a little bigger when it is small. That's the reason why only the average
distance between the precipitates is important since it governs the amount of bowing necessary before a loop can be formed.
In real life this looks like this: |
|
| |
| |
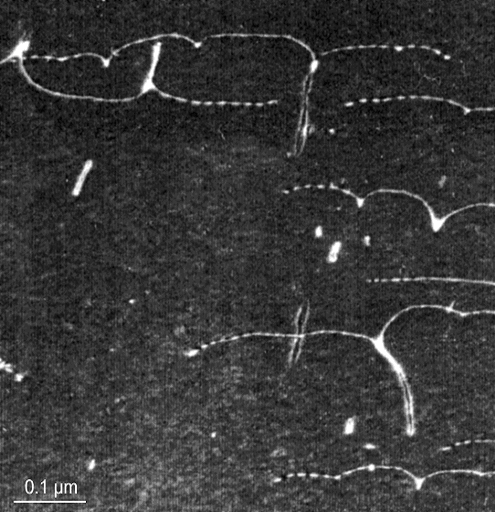 |
| Dislocations (white lines) in a Ti-Al alloy stuck at precipitates (TEM) |
| Source: Picture taken by A. Appel (GKSS Geesthacht); by permission. |
|
| |
| |
|
 |
The precipitates are not visible at the relatively low magnification. Some dislocations are
majorly stuck, pulling out long trails.
It is not so easy to see "stuck" and bowed dislocations in a transmission electron microscope (TEM) image because they usually become unstuck
during stress release and specimen preparation, and all you see are straight lines.
The white dots are dislocation
loops surrounding precipitates that were left back |
|
 |
After the crystal has gone through all the early stages of precipitation formation, only the
Orowan mechanism is left to obstruct the dislocation movement. It doesn't matter what kind of precipitates we have, and
the hardness now comes down with time as shown since the density decreases due to Ostwald ripening, leading to an increase
in the average distance and thus to a decrease in hardness. |
 |
The total hardness vs. time curve then is just the superposition of all the individual
mechanisms working in parallel. Obviously, the best one can achieve is to have early Q''
precipitates plus a few GP zones that are still around. |
 |
It is clear that the major task is to calculate the development of the structure.
What kind of mix of solute atoms, GP, zones, Q precipitates of all kinds do we have at
some specific time t, with all the concentrations and average sizes? It is not an easy task because everything
is coupled to everything else: |
|
 |
The density rprec and the average spacing <l>
of one kind of precipitate is directly related; average size and density determines how many atoms are involved; the degree
of supersaturation gives you the maximum number of atoms you can precipitate, and the difference between the actual number
of atoms in precipitates to the total number tells you what can go on besides the precipitation you are looking at. The
value of the temperature tells you how fast atoms can move by diffusion and thus how fast
things can happen. In addition it gives you an idea about the magnitude of the driving forces that tell you how urgently things should happen.
|
|
 |
If we have the basic data (in particular about the relation between matrix and alloy atoms
and diffusitivies) we can come up with a system of coupled differential equation and solve them. That's not all that easy
with pencil and paper but no problem with computers. I gave you an example about something similar (though far easier) before. |
|
 |
In essence, we need to calculate the rate of nucleation,
how many nuclei form per second, and how fast they grow. The two processes involved
are opposed: At low temperatures you form a lot of nuclei per second, but they grow very sluggishly due to slow diffusion.
At high temperatures it is the other way around. This gives us already a hint that you want to go for some special medium
temperature if you want things to happen fast.
I look at this in detail in another
module and won't discuss it here anymore. The catch word is "time-temperature-transformation"
or TTT diagram |
| |
| |
| |
The Aluminium - Aluminum Controversy |
 |
The metal was named by the English chemist Sir Humphry Davy in 1808 when he was
trying (unsuccessfully) to isolate it from the mineral alumina, a name given by the English chemist Joseph Black
in 1790 to what french called alum, the German Alaun, and the old Romans alumen and modern chemists KAl3[(OH)6|(SO4)2].
It's a white mineral that had been used since ancient times for dyeing and tanning, among other things.
Sir Humphry
knew that some not yet discovered element was hiding in there: "Had I been so fortunate as to have obtained more certain
evidences on this subject, and to have procured the metallic substances I was in search of, I should have proposed for them
the names of silicium, alumium, zirconium, and glucium", he wrote in the Philosophical
Transactions of the Royal Society of London in 1808.
Note that he used yet another version in this first try. He changed
his mind, however, and went from alumium (in 1807) to aluminum and finally, influenced by learned colleagues,
to aluminium in 1812. The -ium ending just was more musical and went well with the -ium
endings of other elements like potassium (K; from German "Kalium"), sodium (Na, from German natrium), magnesium
(Mg), calcium (Ca) or strontium (Sr).
The Danish physicist and chemist Hans Christian Ørsted might first have
produced Al in 1825 in an impure form. This seems not be absolutely certain, however, so Friedrich Wöhler, a German
guy who definitely produced aluminium in 1827, is also credited with the discovery. |
|
 |
In the USA, some dictionaries stuck to "alumium" but nobody gave a
damn as along as Al was extremely rare and more expensive than Gold (Au). Shortly before the 1900 millennium the metal began
to be widely available and the word started to be needed in popular writing. The USA eventually settled on aluminum, the
rest of the world mostly went for aluminium
The American Chemical Society only adopted "aluminum" in 1925,
in response to the popular shift that had already taken place.
The International Union of Pure and Applied Chemistry
(IUPAC), the top authority on this, officially standardized on aluminium in 1990. The people in the US, of course, have
totally ignored that decision. So in 1993 IUPAC grudgingly also accepted "aluminum" and that's where we stand
today. |
 |
Aluminium is the third most abundant element after oxygen and silicon. That makes
it the most abundant metal in the Earth's crust. It makes up about 8% by weight of the Earth's solid surface.
But Aluminium,
like silicon, is very reactive and therefore never found in an elemental stage. It is not easily induced to give up its
partners and belongs to what I have termed the electro-smelting of very difficult metals age. |
| |
|
© H. Föll (Iron, Steel and Swords script)








