| |
4.4 Inside a Perfect Crystal |
| |
4.4.1 Perfect Crystals and the Second Law |
 |
Let's take a hypothetical perfect iron crystal (a brain crystal, in other words), or just any perfect crystal, for
finding out what crystals will do when you heat them up. |
|
 |
No real iron crystal will ever come close
to being perfect, but the silicon
crystals grown for microelectronics actually are not so bad. Whatever, it's no problem for us here. We work with a "brain-and-paper
crystal" and it's ridiculously easy to keep those perfect. |
 |
First we look at our crystal at very low temperatures, close to absolute zero
(=0 K). It's not so easy to do this in reality but it's no real problem either if you have the right equipment. |
|
 |
Disorder then hardly counts for achieving niravana. Whatever disorder or entropy S you have,
the product TS at very small temperatures close to zero is always very small; for T=0 K it would be zero, no matter how
large S would be |
|
 |
So coughing up some energy to produce disorder doesn't make any sense when
it's really cold. The return on that investment cannot be large. It is thus clear that whenever it's very
cold, you thrive for perfect order as shown on the left in the (schematic!) figure below. |
| |
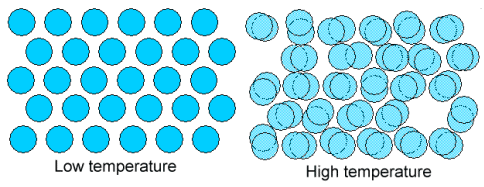 | | Law and order for crystals |
| Schematic drawing of a perfect crystal at very low temperature. Perfect order, with atoms sitting (almost) still.
|
Same crystal at high temperature. Atoms are vibrating wildly (hard
to draw) and two vacancies have formed. |
|
 |
Now we increase the temperature a little
bit. What is our crystal to do? |
|
 |
To answer that question, we first have to answer that particular question that kept nagging
you subconsciously all this time, giving wild dreams about hot things and keeping you from sleeping well: |
| |
|
 |
You think that is an easy question? You think that any idiot can tell the difference
between hot and cold water, or a hot and cold day? Allright. Tell me about it! |
|
 |
Aha. Hot water is hot because you burn your fingers when you stick them in. And it's a cold
day when your a.. freezes off, and hot day when you get a sunburn.
Fine, but how can your a.. tell that it's freezing?
And I hope you will excuse my saying so, but science doesn't give a fart about how your fingers or your a.. feels
about temperature.
Considering that you consist mostly of water (and some beer and red wine in my case) the question
is: how do the water molecules in your a.. know that it's cold enough to start freezing? |
|
 |
Remember, all we have so far are atoms, molecules, crystals
and whatever else you can make from atoms, plus photons (the particles of
light), and the first and second law.
Maybe there is something like a "temperature particle"? Let's call
it phlogiston (ancient Greek of course; phlogistón="burning
up"), so we have a name and feel better about it. Having invented phlogiston makes things simple: shove some phlogiston
around and things get hotter or colder. You could also call it "caloric"
and assume it is an element like carbon, if that makes you feel better. The eminent Antoine Lavoisier, the "father of modern chemistry", did that in 1789, not all
that long ago.
Tough luck! There is no phlogiston, or caloricum, or any substance
of heat—notwithstanding the fact that a large part of humankind believed that for quite some time; use the links for
details. Atoms, molecules, crystals and whatever else you can make from atoms, plus photons
(the particles of light) is the complete list. No phlogiston, caloricum, heatstuff or whatever. |
 |
Looking at a perfect crystal you see atoms, and nothing
but atoms! The question then must be: What is different in the arrangement
of atoms at low and high temperature? The arrangement is the only thing that could be different!
Please commit to memory: |
| |
Temperature is just another word for
"average energy contained in the
random movement
of elementary things
like atoms or molecules".
|
|
 |
Let's whip out your brain
microscope and give the air around you a close look. Ready? Then you should see a lot of nitrogen and oxygen molecules
(N2 and O2, respectively) flitting around at high speed and bumping into each other all the time. |
|
 |
The animated figure below shows schematically at very high magification (around 10.000.000×)
and in v e r y s l o w motion what you are supposed to see: |
| |
| | The air as seen with your brain microscope |
Colors are only used to make it easier for you to keep track of some particle.
In real air the spheres would be (elongated) oxygen or nitrogen molecules, there would be far more of them then shown here,
they move on average with the speedof sound, and they would move in three dimensions.
|
| Source: English Wikipedia, author A. Greg |
|
|
 |
The molecules bump into each other (and into the atoms of the wall) all the time, and that
makes their motion rather random. Their total energy then is just the average
kinetic energy of one molecule times the number
of molecules. Their average kinetic energy, of course, is determined by their mass and
average speed squared.
The average random energy
of those atoms or molecules is exactly what we call the temperature of the gas, it just
happens to be measured on a different scale for historical reasons.
Note that the gas molecules in your car, traveling
along with you at whatever speed you are driving at, also have some additional kinetic
energy because of the car motion. This is, however, not a random motion. All molecules
travel with the same speed you are traveling with on top of their random motion—and
this does not count! |
 |
We can measure this kind of energy in "Kelvin" (we never say "degree
Kelvin, by the way), "degree Celsius", "degree Fahrenheit", or whatever temperature
scale you prefer. The numbers are quite different but it is always the same energy.
This might be a bit confusing but it is nothing new. |
|
 |
You can measure your wealth in Dollars, Pounds, Euro, or the number of Ferraris it can buy.
You will produce quite different numbers for each scale, but it is the same wealth and
you can always switch from one number into the other one. |
| |
Converting energy measured in barrels of oil, British thermal units (btu) Joules (J), kilowatt-hours
(kWh), electron volts (eV), or temperature (T) produces quite different numbers too, but it is the same energy and you
I can easily convert from one unit to another one. It's just as easy as switching from one currency to another one, with
the additional advantage that the exchange rates never change. |
 |
By the way, how fast on average are the molecules of air at room temperature?
What's your guess? Faster or slower than your Ferrari at top speed? |
| |
|
 |
Here is the answer: they run around with the speed of
sound, about 330 m/s. |
|
 |
It can't be otherwise because sound is a local disturbance in the running–around pattern
of the gas molecules, and this disturbance cannot possible mover faster than its parts, the air molecules. |
 |
Now whip out your brain microscope once more and look at molten
steel. At the magnification required this can actually only be done with
a brain microscope. It will be rather hot work so, for starters, let's look at a more convenient liquid like water at room
temperature. Beer or red wine is just as good, if that helps your looking.
What are you going to see? |
|
 |
Water molecules in liquid water are flitting around randomly and bumping into each other all
the time! The bumping into each other makes their motion random, and so on. Schematically
it looks exactly as in the animation above.
The animation above could also be used to schematically
illustrate water vapor, a gas. It is pretty much fine for all liquids and gases.
So what is the difference between a liquid like water and a gas
like water vapor?
In a liquid the atoms or molecules are closer together and still stick to each other a bit. If given
more room, they would not thin out like water vapor or any gas but still occupy the same volume. Nevertheless they move
around freely but always feel some attraction to the other guys, not unlike people at a party in a crowded room. They move
around freely but also stick together. |
|
 |
If we extend the analogy from a a party to a crowded movie theatre where people sit on the
same chair all the time, we changed from liquid to a crystal.
Nobody is running around, everybody, including you, sits at a fixed position.
All you can do is to wiggle around that
position a bit. Only people with a vacancy (=unoccupied seat) next to them
can move—one place at a time. |
 |
Now back to our perfect crystal.
In contrast to gases or liquids, the crystal atoms cannot move around to acquire some
of the energy we call temperature. All they can do is to wiggle around their position. They do that very quickly so we don't
call it "wiggle" but oscillating or vibrating.
Those atoms or molecules thus oscillate around their proper position a bit.
The maximal deviation from their position
at rest we call amplitude. The rest is easy:
- Low temperatures=small amplitudes.
- High temperatures=large amplitudes.
|
|
 |
Oscillations or vibrations are hard to draw. I did the best I could in the figures here and
there. What we can see, however, is that the hot crystal above, with its atoms oscillating wildly, would look a lot less orderly than the cold one next to it—even
if there weren't any vacancies.
Vibrations of the atoms evidently induce disorder or, as we agreed
to say, entropy. |
|
 |
Here we have the full answer to the question from (far) above!
When we raise the temperature, the energy goes up, yes - but so does the entropy. At the raised temperature we can always
find a proper balance of energy and entropy and achieve nirwana again. Everybody is happy. |
 |
Now we get our crystal going and raise the temperature a
lot. We give more thermal energy to the crystal, to introduce a new buzz
word. |
|
 |
The atoms now must vibrate wildly, with large amplitudes, and that is part of the reason why
the crystal becomes a bit larger by thermal expansion.
Entropy increases too,
but not enough for nirvana purposes. There is only that much disorder you can make by keeping things not exactly at their
proper place but always nearby. Think of your spice rack. Not having the spices
in alphabetical order but still on the rack produces some disorder or entropy. Allowing
some spice containers to be off the rack for good (they might now be in the refrigerator, the underwear drawer, or God knows
where; it doesn't matter) offers far more possibilities for making things messy. |
 |
In very hot crystals we need more massive disorder for achieving nirvana than
we can make with vibrations, and for that we have to misplace things. |
|
 |
The only things we can misplace are the atoms; we have nothing else. So let's misplace atoms
and create vacancies—it was done already in the figure above.
I have misplaced two atoms. They are gone, no longer with us. They might now be in the refrigerator, the underwear drawer,
or God knows where; it doesn't matter. In their place we now have our first crystal defect,
the vacancy. We are going to become very familiar with these little nothings because
they are important for sword making.
Since we cannot really loose atoms completely, you might wonder were they really are now. Well, consider for the time being that they went to that mysterious place where
the missing single socks go. Like those socks our atoms have not
really disappeared into thin air. Your wife could find your single socks for you, I could find the missing atoms for you.
I will eventually—but you don't need to care about
that here; it is irrelevant for what I want to get across here. |
|
 |
Our crystal looks really messy now and we have plenty of entropy. For making
the vacancies, as we will call the missing
atoms now, we had to invest some energy but as a return on the investment we got substantial entropy.
If we
invest just right, we make exactly the right amount of vacancies to give the best balance between energy and entropy for
the temperature we have chosen. We have achieved nirvana once more. |
 |
"What the hell", you might be inclined to yell out now. "Wen I
drive around with my girl friend in search of a vacancy in some motel, I might care about vacancies. This here looks like
some crazy little game for academically inclined eggheads but certainly does not relate in some important way to my girl
friend or to making a sword." |
|
|
Well, you're wrong. Utterly wrong.
|
|
|
 |
Without vacancies in crystals you would not drive
in a car. You might not even exist, because Columbus would not have discovered America. The likes of you would live in what
we call the stone age. |
| |
|
© H. Föll (Iron, Steel and Swords script)

