| |
4. Iron, Crystals, and the Second Law |
| |
4.1 A Close Look at Real Iron |
| |
4.1.1 Microscopes |
 |
Get a piece of iron. Any kind of iron, including "mild steel"
with just a little bit of carbon. In case of doubt, cut off a piece of your car body or take a regular nail. |
|
 |
Now look at it. Not just so but with magnifying glasses or a good microscope.
You don't have one? No matter - just look with your brain not with your eyes. It
is easier and far cheaper. |
|
|
Important News:
Thanks to me you now own a powerful
brain microscope.
You can crank up the magnification
to at least ten million.
|
|
|
 |
Ten million magnification power! That's well
beyond the magnifying power of a standard-issue optical microscope. Those will give
you about thousand-fold magnification at best. |
 |
If you're not satisfied with your personal brain microscope
at ten million magnification (all brains are not equal, after all), you need to buy
a so-called "High Resolution Transmission Electron Microscope",
or HRTEM for short, if you want to be able to keep up with your peers. |
|
 |
The mighty transmission electron microscopes
are not to be confused with the lowly "Scanning Electron Microscopes"
or SEM.
Those can only show you the surface of your specimen at magnifications rarely exceeding
a few hundred thousand. With a HRTEM you can actually look inside the specimen at magnifications
exceeding 10 million.
If you want very high magnification or resolution but are satisfied to see just the surface
of your iron, you may want to get a "Scanning Tunneling Microscope"
or STM. |
| |
 |
Microscopes are not exactly cheap. A simple run-of-the-mill optical microscope,
the kind of microscope all those movie actors pretending to be weird scientists with a German accent are always looking
into, slightly frowning because they don't have the faintest idea of what they are doing, will only set you back 50.000
$ to 100.000 $. Those cheapies will also be of no use for what we are about to do.
You need to cough up at least 3 Million
$ for a decent HRTEM if you're not satisfied with your brain microscope. A decent SEM will be around 600.000 $. Simple STM's
you may get already at a bargain price of a few 100.000 $; better ones will take a million or so |
|
|
 |
If you can come up with that kind of cash, figuring perhaps that some fancy hardware
is much showier than your brain anyway—think again. There is always the first law of economics, remember? |
|
 |
There is no such thing as a free lunch, particularly in science.
Even if you can pay for the microscopes, you won't understand the manual. You must spent at least 5 years of your life (full
time) for learning how to work your microscopes. |
 |
You can avoid all this expense of money and time if you just use your "brain
microscope". All you have to do is to imagine what there is to see. I
will help you by telling you what it is that you see. |
|
 |
Of course, if you already know what you will see, you don't have to read on. But
changes are you don't, in contrast to me. OK—I will now help you to operate your brain microscope. First you need to
turn it on. |
|
|
|
 |
What are you going to see if you look at your piece of iron at very high magnification
with your brain microscope? Given your halfway decent education, you should know this without me telling you! |
|
 |
OK—since you're still with me, I'm going to tell you. You see: |
| |
|
|
 |
What did you expect? Everything you can see: the stars in the sky, your wife, the TV set, the mountain
range, the ocean, your boss or your underlings, the dust mites dancing in a ray of light, everything—and that includes
you—consists of atoms and photons. |
|
 |
That's it. There is nothing else for non-scientists like you. It's my and my
fellow scientists privilege that on rare occasions we can also "look" at things that are different from atoms
(including their constituents) and photons. We have, for example: positrons, muons, quarks and gluons,
or some other weird elementary particles.
We have to make those guys with great effort (and plenty of your, the tax-payer's money), and they usually stick around
for only rather short times.
So forget them. Those particles need not concern you at all beyond paying our bills.
You might object and say that if you want to make and run a decent universe,
you also need gravitation. But that is "just" a property that all atoms and photons
carry around with them, and it need not concern us at all as long as we study a piece of iron or steel that is considerably
smaller then a small moon. | |
|
 |
What needs to concern you besides atoms is only the photon,
the "particle of light". |
|
 |
If you have learned that light is not a particle
but an electromagnetic wave, that's fine. The photon as an object
of quantum theory encompasses all of that wave stuff too, and that's why we don't need
to worry about electricity and magnetism here either. It's all in the photon. Light is not just a wave or just a particle,
it is light. It was always very difficult to explain to non-physicists what is meant by photon or wave. But now we have
a new word: Waves or particles are avatars of light. The represent some but not all
aspects of the real thing. |
 |
We really need the photons so we can see. But they have many other uses too, because
they come in many kinds. | |
|
|
 |
The light that our eyes can perceive consists of photons, and so does the infrared
or ultraviolet light that some animals can see (and my colleagues and I with proper instrumentation). The microwaves heating
your food consist of photons, and so do the TV and radio waves that soften your brain after prolonged exposure. Of course
that's not done by the photons directly, to be sure, but by the content they were forced to transport.
Photons also
come as X-rays and Gamma rays. In this case they are produced by proper machinery, decaying
atoms, or exploding stars. All electromagnetic radiation consists of photons; they are
only differentiated by their energy. | |
 |
In case you wondered: I haven't mentioned the electrons
that we obviously need for an electron microscope because I don't need to. |
|
 |
Electrons are part of atoms. If we have atoms,
we have electrons. All we need to do is take one out of its atom by being a bit brutal (remember the first law of materials science?) The sorry atom, bereaved of one electron, we then call a
positively charged ion but we needn't really to know this for what follows. |
 |
We have a big thought here, probably the biggest there is in
science: |
|
|
All that exists around us (including us)
consists of atoms and photons.
|
|
|
 |
There are about 90 different atoms (arranged in the periodic
table) that we might encounter when we are looking at things at very high magnification. As long as we look at our piece
of iron, we are going to see iron atoms, and possibly an occasional carbon (C) or manganese (Mn) atom if we have mild steel. If we look very
closely, we find the occasional atom of everything else, too. |
 |
What does a single atom, iron or otherwise, look like?
|
|
 |
Nobody knows that from looking at one. You simply can't see it. Not for lack of
magnification of your microscope but because you cannot hold a single atom in the proper position inside your microscope.
If you just put it there and then let go, it will run off.
Also consider: how would you get it there in the first place?
All you have to manipulate your single atom with are other atoms. |
 |
You now get the idea: We can look at a single atom only
when it sits on the surface of a solid formed by a bunch of other atoms like in this
picture.
Alternatively, we can look at a whole bunch of atoms that constitute a solid if, in the direction we are
looking, they sit exactly on top of each other. |
|
 |
If we make our iron specimen thin enough so the electrons of our HRTEM can pass
right through, we can put it inside the microscope and keep it in a fixed position oriented in such a way that we look at
a whole column of atoms. |
|
 |
So if you crank up the magnification of your HRTEM to about 10 million fold,
you are going to see atoms as whitish blobs on your screen. What we are going to see below are gallium
(Ga) and arsenic (As) atoms arranged into a crystal
called Gallium arsenide (GaAs). |
| |
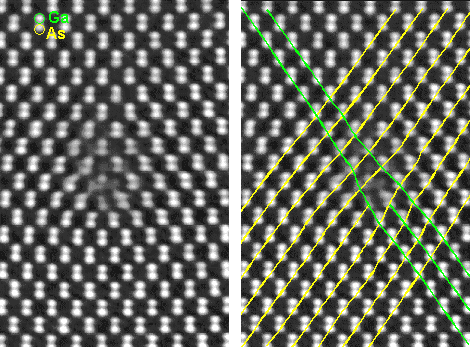 |
| HRTEM picture of columns of Ga and As atoms in a GaAs crystal |
The lines in the left picture are just to guide the eye. The crystal
obviously contains a defect.
|
| Source: from my friends at the KFA Jülich, Germany |
|
|
 |
This is one of the better pictures of atom (columns) presently available, taken
in the "KFA Jülich"; a large German research institution that was instrumental in developing a new brand
of HRTEM's with superior performance.
Every white blob is either a row of Gallium (Ga) atoms or arsenic (As) atoms standing
exactly edge-on. I use a picture of Gallium arsenide and not of iron to demonstrate my point because iron pictures are not
quite as nice for some technical reasons that are not important here. |
|
 |
The picture nicely illustrates two important points:
- The atoms of the substance gallium arsenide (GaAS) are arranged in an orderly pattern.
If you have seen a few, you have seen them all. Expressed in other words: if you have seen a few columns of atoms, you know
exactly how to continue their arrangement. Gallium arsenide, in other words, is a crystal.
- There is some disturbance of the orderly pattern in the center of the picture. Our crystal contains a crystal defect. Drawing some lines that connect neighboring atoms as done in the right
part reveals the nature of this defect: it is a dislocation.
Jump ahead if you want to know now
what a dislocation is.
|
 |
The two "important points" expressed
by the buzz words: "crystal" and "crystal defect" are actually so important that we will spend this
and the next chapter to look into crystals and their defects in some detail. Your sword
blade, by the way, is a crystal. It has the properties it has because of the defects in that crystal. |
|
 |
Oh, I almost forgot: Gallium arsenide (GaAs), a substance you probably never have
heard off, is not just something Materials Scientists use to test their microscopes with. It is nothing less but the paradigmatic
material for optoelectronics, the father and mother of a group of semiconductors that
include materials like Gallium aluminium arsenide (GaxAl1-xAs), Gallium nitride (GaN) or Gallium phosphide
(GaP).
So what is optoelectronics? If you ever used a Laser, e.g. in your DVD player, a light
emitting diode (LED),
or optical communication via glass fibers, you used optoelectronic products. No more needs to be said. |
| |
| |
© H. Föll (Iron, Steel and Swords script)
