 |
Now you may be tempted to write this down in a first reaction equation
as follows |
| |
|
|
 |
While this is not necessarily wrong, it is at least strange: You create, in a kind of chemical
reaction, something from nothing - what keeps you from applying this equation to vacuum, which is surely not sensible? Maybe
you should somehow get the crystal involved as the reference system within which things happen? |
 |
So let's device a more elaborate system by looking at our crystal before
and after a Frenkel pair was formed |
|
 |
Before a Frenkel pair is formed, the site occupied by
the vacancy after the formation process is a Na site, we denote it by NaNa.
This simply means that a Na atom occupies a Na site before a vacancy is formed there. |
|
 |
At the interstitial site, where the Na interstitial after
the formation process is going to be, you have nothing before the process. However,
all those possible interstitial sites also form a lattice (e.g. the lattice of the octahedral
sites); in a perfect crystal all those sites are occupied by vacancies, we consequently
denote an empty interstitial site by Vi = vacancy on an interstitial site. |
|
 |
A Na ion on an interstitial site then is Nai, and a Na vacancy
becomes VNa . Now we can write down a reaction equation that reads |
| |
|
 |
This
looks like a cool reaction equation, we now create a Frenkel pair within a
crystal and not out of thin air. |
|
 |
Indeed, the reaction equation does look
so much better this way! Small wonder, we just invented part of the so-called Kröger-Vink notation, in use since the fifties of the 20th century - not all that long ago, actually.
|
|
 |
This notation is also called notation by structure elements
and it is very useful for formulating all kinds of reactions involving point defects. However, the first
law of economics applies ("There is no such thing as a free lunch"): |
| |
Don't use the mass
action law uncritically with these kinds of reaction equations!. The reason for this is simple,
but usually never mentioned in the context of chemical reaction formulation: |
| |
| A proper reaction equation contains only
reaction partners that are independent |
|
|
 |
This means that you can, in principle, change the concentration of every
reaction partner without changing the others. |
 |
Consider for example the following purely chemical simple reaction equation: |
| |
|
|
 |
You can put arbitrary amounts of all three reaction partners in a container and change any
individual amount at will without changing the others. |
 |
In our reaction equation for point defects, however, you cannot
do this. If you consider, e.g., to change the NaNa concentration a little, you automatically
change Vi, too - those quantities are not independent! |
|
 |
This was the bad news about using Kröger-Vink notation. The good news are: In most practical
cases it doesn't matter! Chapter 2.4 - often alluded to - will contain details about all of this.
|
 |
It is not easy to grasp the reaction equation concept for point defects in all
its complexity, but it is worthwhile if you want to dig deeper into point defects. For the purpose of this paragraph let's
just postulate that the two sums left and right of the reaction equation would constitute
the proper reactants (those sums, by the way, are called building elements in the
Schottky notation). |
|
 |
Be that as it may, we now apply the mass action law, keeping in mind that
the reaction equation from above in full splendor actually contains a reaction enthalpy GReaction,
i.e.: NaNa + Vi + GReaction Û Nai
+ VNa) |
| |
[NaNa] · [Vi]
[Nai] · [VNa] |
= const = exp |
GReaction
kT |
| [Nai] · [VNa] | = |
[NaNa] · [Vi] |
· exp – |
GReaction
kT |
|
|
|
 |
GReaction, of course, is the free enthalpy change of the crystal
upon the formation of one mol of Frenkel pairs. If we relate it to 1
Frenkel pair, it becomes HFP. |
|
 |
The [...] are the molar concentrations of the respective
quantities if we use molar reaction enthalpies. |
|
 |
OK, now let's spell it out. If we have a crystal with N
mols of NaCl, we have the molar concentration
of [NaNa] = N for really obvious reasons. |
|
 |
[Vi] = N, most likely, will hold, too, but here we may have to dig
deeper. How many different places for interstitials do we have in the given unit cell? We can figure it out, but for the
sake of generality their molar concentration could be larger or N' - so it can be different in principle from
N as we have seen before. |
 |
Since we usually go for atomic concentrations, we note that cV(C)
= atomic concentration of the cation-vacancy = [VNa] /N and
ci(C) =
atomic concentration of the cation-interstitial = [Nai] /N
we now obtain one equation for the two unknowns
cV(C) · and ci(C), which will be the first
of the equations we will need for what follows. |
|
|
| cV(C) · ci(C) |
= | N'
N |
· exp – |
HFP
kT |
| (1) |
|
 |
Note that this is
not our old result, because it does not imply
that cV = ci. All the mass action law can do is to supply one
equation for whatever number of unknowns. |
 |
We need a second independent equation. This is - of course (?) - always electroneutrality.
Looking just at Frenkel pairs, we have directly |
|
|
| cV(C) | = |
ci(C) | | | |
| | for Frenkel Pairs only |
|
|
|
 |
Now we have two equations for two unknown concentrations that we could easily solve. |
 |
However, we are interested in mixed defects
here, so we must also consider Schottky defects and then mix them with Frenkel defects,
always maintaining electroneutrality. |
 |
We might now go through the same procedure as before by using
a similar reaction equation for Schottky defects - with a few more complications in finding the proper reaction equation.
We will not do this here (do it yourself or use the link), just note the rather
simple result: |
|
 |
With cV(A) and cV(C)
denoting the vacancies on the anion or cation sublattice, resp., and with HS = formation enthalpy
of a Schottky pair, we obtain for a second equation |
| |
| cV(A) · cV(C) |
= exp – |
HS
kT |
| (2) |
|
 |
Again, this is not the old equation
for Schottky defects - the concentrations are not necessarily equal once more |
|
 |
Note that the vacancies on the anion or cation sublattice are positively
or negatively charged - opposite to the charge of the (negatively
charged) anion or (positively charged) cation that was removed! A cation
vacancy thus carries a negative charge and so on, whereas a cation interstitial carries a positive charge. Look at the
illustrations if you are not clear about this! |
 |
Knowing that electroneutrality has to be maintained (look at the direct calculation for Schottky defects), we introduce electroneutrality now for the more general
case of our three charged defects: The sum of all charges on the point defects must
be zero; we obtain the third equation |
|
|
|
|
 |
Or:
Sum over all negative charges = Sum over all positive charges. |
 |
Now we have 3 equations for 3 unknown concentrations,
which can be solved with ease (haha). We obtain for the general situation of mixed defects |
| |
| | cV(C) |
= exp – |
HS
2kT |
· |
æ
ç
è |
1 + | N'
N |
· exp |
HS – HFP
kT | ö
÷
ø
| 1/2 |
| | cV(A) |
= exp – |
HS
2kT |
· |
æ
ç
è |
1 + | N'
N |
· exp |
HS – HFP
kT | ö
÷
ø
| – 1/2 |
| | ci(C) |
= | N'
N |
· exp |
Hs
2kT |
· exp – |
HFP
kT | ·
| æ
ç
è |
1 + | N'
N |
· exp |
HS – HFP
kT | ö
÷
ø
| – 1/2 |
|
|
|
 |
These equations contain the "pure" Frenkel and Schottky case as limiting cases. |
 |
Was that worth the effort? Probably not - as long as you just look at simple ionic
crystals (where one defect type will prevail anyway). being in simple equilibrium without considering surfaces and the environment. |
|
 |
However! In real life, where point defects in ionic (and
oxide) crystal are used for sensor applications, this kind of approach
is the only way to go! It will be far more complicated, there will be approximations
and "short-cuts", but the basic kind of reasoning will be the same. |
 |
Now it is time for an exercise |
| |
|
| |
 |
We now have the general equations and thus can answer the essential question for
this case: How likely is a mixed case? |
|
 |
In a more quantitative fashion we ask: How different must the formation enthalpies be if just
one defect type should dominate? |
|
 |
That is, of course, an excellent exercise question. |
| |
|
 |
If you do the exercise, you will find out that relatively small differences in
the order of 0.1 eV in the formation energies of Schottky or Frenkel defects will already lead to the preponderance
of one defect type. |
|
 |
This is important! Since it is quite likely that formation enthalpies for Schottky or Frenkel
defects in a given crystal will differ by more than just 0.1 eV, we are quite justified to look at the defect situation
in an "either - or" mode: "Either we will have Frenkel defects, or Schottky defects. One kind will practically
always "win". |
 |
The final questions are then: When do we have Schottky disorder, when do we have
Frenkel disorder? What are typical formation enthalpies? Are there general criteria for what kind is more likely to occur
in a given crystal? Can we tell or at least make an educated guess? |
|
 |
Well let's look at a few experimental findings (all numbers are from "Hayes and Stoneham" ; more numbers can be found in the link): |
|
|
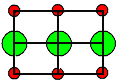
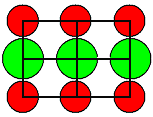
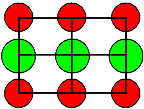
| Crystal | Type of lattice | Type of disorder |
Formation enthalpies HS/F [eV] |
<110> projection
Ion size to scale |
Cations = red
Anions = green |
NaCl
and all other alkali halides |
NaCl-type |
Schottky |
LiF | 2,5 |
 projection" TITLE="click to enlarge size">
projection" TITLE="click to enlarge size"> | | NaCl | 2,3 | |
| KCl | 2,3 | | | KBr | 2,4 | |
| CsI | 1,9 |
 projection" TITLE="click to enlarge size"> projection" TITLE="click to enlarge size"> |
| Some Oxides | MgO | 5,7 | | AgCl
and AgBr, |
NaCl-type
|
Frenkel
with cation interstials | AgCl | 1,45 |
 projection" TITLE="click to enlarge size">
projection" TITLE="click to enlarge size"> | | NaNO3, KNO3, .. |
complex | | | |
CaF2
and SrF2, BaF2 |
CaF2 type |
Frenkel with
anion interstials
("Anti-Frenkel") |
CaF2 | 2,7 | | | SrF2 | 2,3 |
| | BaF2 | 1,9 |
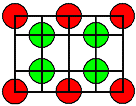 projection" TITLE="click to enlarge size">
projection" TITLE="click to enlarge size"> | | SrCl2 | 1,7 | |
|
 |
What we see is: |
|
 |
1. It is hard to make a prediction of what kind of defect you will find. Even looking
at a lattice projection with the ions drawn to scale (using tabulated
ionic radii) does not offer immediate clues (besides the obvious one that you only would expect Anti-Frenkel
defects with anion interstitials, if the anion is not much larger than the cation). |
|
 |
2. The formation enthalpies of Frenkel defects tends to be a bit lower than those of
Schottky defects. |
|
 |
3. The formation enthalpies in the more simple ionic crystals (no oxides) tend to be
rather small - around 1 eV per single defect; quite comparable to the vacancy formation enthalpy of simple metals.
This might be taken as a hint that charge matters less than bonding and lattice distortion. |
| |
|
© H. Föll (Defects - Script)