 |
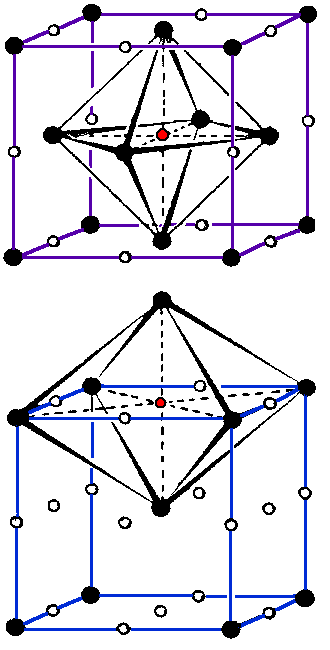
| An octahedral position for an (interstitial) atom is the space in the interstices between 6 regular atoms that form an octahedra. | |||||
| Four regular atoms are positioned in a plane, the other two are in a symmetrical position just above or below. All spheres can be considered to be hard and touching each other. | |||||
| The six spheres define a regular octahedra, in its interior there is a defined space for an interstitial atom, bordered by six spheres. | |||||
| Octahedral sites exists in fcc and bcc crystals. The other prominent geometric environment for interstitials is the tetrahedral site. | |||||
| This illustration shows the octahedral site in an fcc lattice bottom. We have 12/4 +1 = 4 positions per unit cell. | ||||
| Here we have octahedral sites in the bcc lattice. We have 12/4 + 6/2 = 6 positions per unit cell. | |||||
![]() 1.3.3 The larger View and Complications
1.3.3 The larger View and Complications
![]() 2.1.1 Simple Vacancies and Interstitials
2.1.1 Simple Vacancies and Interstitials
© H. Föll (Defects - Script)