 |
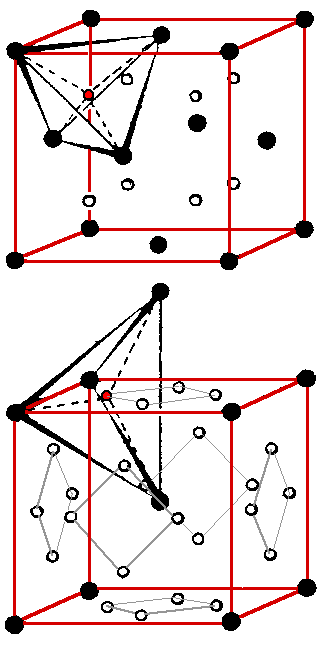
In a tetrahedral site the interstitial is in the center of a tetrahedra forms
by four lattice atoms. Three atoms, touching each other, are in plane; the fourth atom sits in the symmetrical position
on top. |
|
 |
Again, the tetrahedral site has a defined geometry and offers space for an interstitial atom. |
| | |
| |
|
 |
The configuration on top is the tetrahedral position in the fcc lattice. The black circles denote
lattice points, the red circle marks one of the 8 the tetrahedral position. |
| | |
 |
The picture on the bottom shows the tetrahedral configuration for the bcc lattice. We have (6
· 4)/2 = 12 positions per unit cell. |
© H. Föll (Defects - Script)
![]() 1.3.3 The larger View and Complications
1.3.3 The larger View and Complications