 |
Interstitials are all atoms sitting not on their regular place, but between other
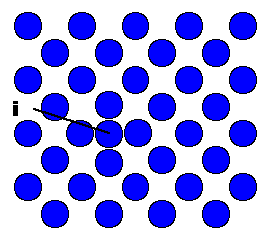
atoms. The picture shows the simple case of a self interstitial atom in an elemental fcc crystal. |
| |
|
|
 |
If the crystal is viewed as periodic arrangement of hard spheres, interstitials sit in the
interstices between the spheres. For the most prominent simple crystals there are two kinds of interstices: Octahedra- and Tetrahedra interstices
or gaps. |
 |
There are two basic kinds of interstitials: Intrinsic and extrinsic interstitials: |
|
 |
Intrinsic interstitials are interstitials atoms
of the same kind as the atoms of the crystal "self-interstitials"). They
are practically non existent in elemental crystals (i.e. in all metals) with the big exception of Si, where intrinsic
interstitials play an important role in diffusion and microdefect formation. |
|
 |
Extrinsic interstitials are interstitial atoms
of a foreign (extrinsic) type, e.g. C in Fe or O in Si. They may diffuse directly through the
lattice (i.e. without the help of vacancies) and play an important role in many
technically relevant materials. |
|
| |
© H. Föll (Defects - Script)
![]() 1.3.3 The larger View and Complications
1.3.3 The larger View and Complications