|
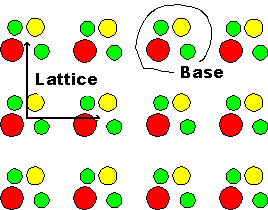
An ideal crystal is a repetition of identical structural units in three dimensional
space. The periodicity is described by a mathematical lattice (which are mathematical points
at specific coordinates in space), the identical structural units (or base of the
crystal) are the atoms in some specific arrangement which are unambiguously placed at every lattice point. Note that a lattice is not a crystal, even so the two words are often used synonymously in colloquial language,
especially in the case of elemental crystals where the base consists of one atom only. |
|
 |
All possible lattices can be described by
a set of three linearly independent vectors a1, a2, and
a3, the unit vectors of the lattice. Each lattice point than can be reached by a translation
vector T of the lattice given by |
| |
| T | = |
(u · a1, v · a1, w ·
a3) |
|
|
|
 |
With u, v, w = integers. |
 |
It is convenient, to classify lattices according to some basic symmetry groups.
This yields the 14 Bravais
lattices
, which are commonly used to describe lattice types. Their basic features are shown below (For sake of clarity, the
lattice points are shown as little spheres and occasionally only "visible" lattice points are shown. These are
not atoms, however!) |
| |
Name of crystal system
Length of Base vectors | Angles
between axes | Bravais Lattices |
Cubic
a1= a2 =
a3 |
a = b = g
= 900 |
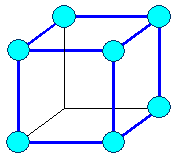
cubic primitive |
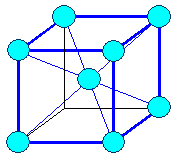
cubic body centered (bcc ) |
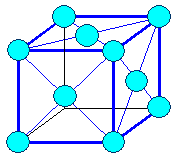
cubic face centered (fcc) |
Tetragonal
a1= a2
¹ a3 |
a = b = g
= 900 |
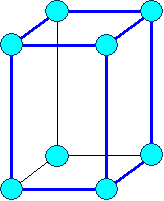
Tetragonal
primitive |
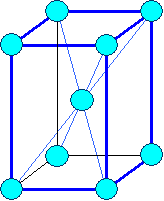
Tetragonal body centered |
|
Hexagonal
a1= a2
¹ a3 |
a = b = 900,
g = 1200 |
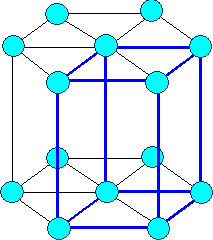
Hexagonal (elementary
cell continued to show hex. symmetry) |
|
|
Rhombohedral
a1= a2
= a3 |
a = b = g
¹ 900 |
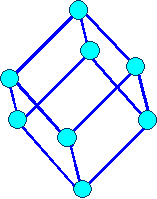
Rhombohedral |
|
|
Orthorhombic
a1
¹ a2 ¹ a3 |
a = b = g
¹ 900 |
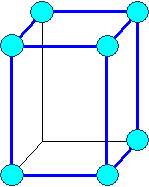
Orthorhombic
primitive |
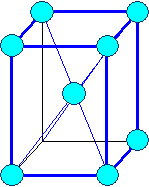
Orthorhombic body centered |
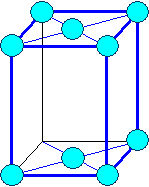
Orthorhombic base face centered
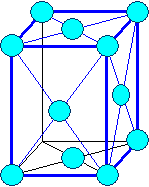
Orthorhombic face centered |
Monoclinic
a1
¹ a2 ¹ a3 |
a = b = 900,
g
¹ 900 |
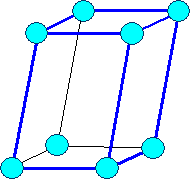
Monocline
primitive |
|
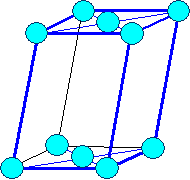
Monocline base face centered |
Tricline
a1
¹ a2 ¹ a3 |
a ¹
b ¹ g
¹ 900 |
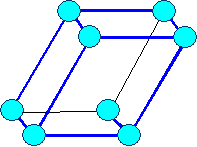
Tricline |
|
|
|
 |
A
crystal now is obtained by taking a Bravais lattice
and adding a base! |
|
 |
The base can just be one atom (as in the case of many elemental crystals, most noteworthy
the metals), two identical atoms (e.g. Si, Ge, C(diamond)), two different atoms (NaCl, GaAs,
...) three atoms, ... up to huge complex molecules as in the case of protein crystals. |
|
 |
An arbitrary example is shown below |
| |
|
 |
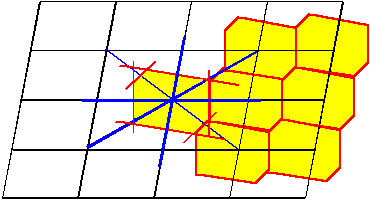
For certain applications, a Bravais lattice may not be the best choice. Whereas,
for example, it shows best the cubic symmetry of the cubic lattices, its elementary cell
is not a primitive unit cell of the lattice, i.e. there are unit cells with a smaller
volume (but without the cubic symmetry). For other cases (especially if working in reciprocal lattices) the choice of a
Wigner-Seitz
cell may be appropriate, which is obtained by intersecting all lines from one lattice point to neighboring points at half
the distance with planes at right angles to the lines |
|
 |
This is shown schematically below: The blue lines connect lattice points, the red lines denote
the intersection at right angles. The resulting Wigner-Seitz cell and its use in constructing the lattice are shown in yellow. |
| |
|
 |
In practical work, one oftens refers to crystal types instead of lattices
by using the name of prominent crystals, crystallographers or minerals etc.; e.g. "diamond type, Perovskites, "Zinkblende"
structure and so on. A few examples are given in the link. |
|
|
© H. Föll (Defects - Script)