To calculate the chemical potential \(\mu_i\) of one component \(i\) we combine Eq. (3.78) and \(\mu = \Delta G_m\)
This is Dalton’s law. Normally \(p_{tot}\)
and \(p^0\) (the standard pressure) are selected 1 bar, thus, the second ln-term is vanishing.
For an extensive property \(\Phi\) the difference by mixing compared to the pure components is
|
| \begin{equation*} \Delta_{mix} \Phi^{id}=\Phi^{id}(mixture) - \sum_i n_i \Phi^{id}_m(pure) \label{eq:DmixPhi_ideal} \end{equation*} | (5.2) |
Since no interaction between the ideal gas molecules exist we find
|
| \begin{equation*} \Delta_{mix} H^{id} = \Delta_{mix} V^{id} = 0 \label{eq:DmixH_ideal} \end{equation*} | (5.3) |
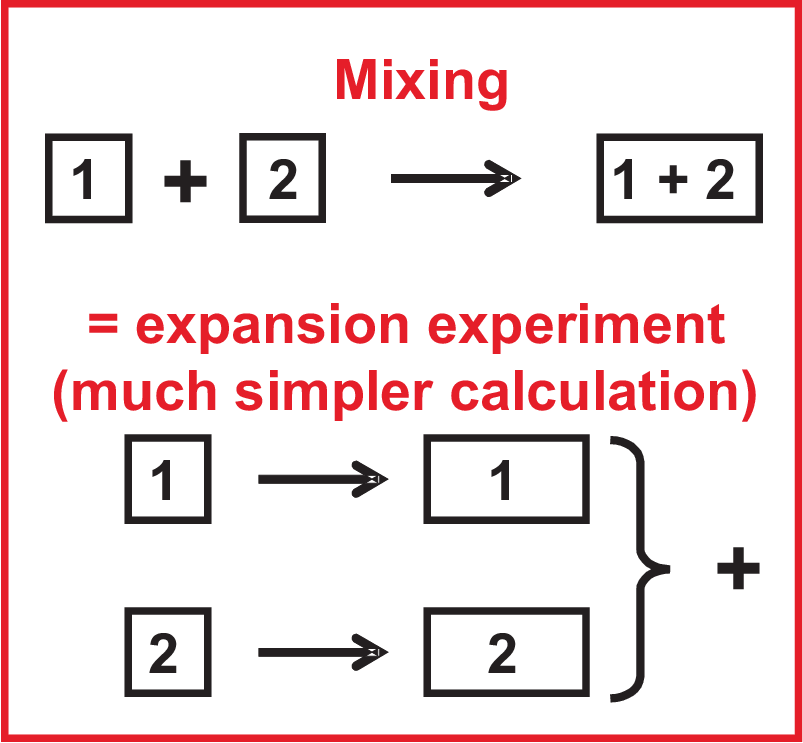
To calculate the effect of mixing on the entropy we apply the scheme of Fig. 5.1. Since \(S\) is a state function we can replace the calculation of mixing by expansion performed separately for each component. Since this ideal mixing is isothermal (\(0 = dU = T \,dS - p\, dV\))
|
| \begin{equation*} dS = \frac{p}{T} dV = \frac{n\,R\,T}{VT} dV = n\,R\, d\ln V \label{eq:dS_isothermal_2} \end{equation*} | (5.4) |
so
Since \(\Delta_{mix} S \gt 0\) and \(\Delta_{mix} G \lt 0\) mixing of gases is SPONTANEOUS, i.e. an irreversible process, even for ideal gases for which no energy contributions exist.
© J. Carstensen (TD Kin I)