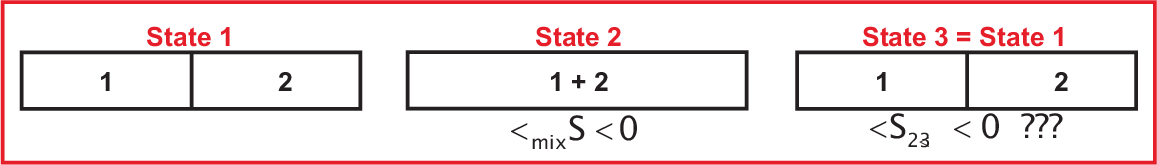
The result of Eq. (5.5) holds independent of the nature of the molecules, i.e. \(\Delta_{mix} S_2^{id}
= - \sum_i n_i \, R \ln x_i \gt 0\). So we can apply this equation on identical ideal gases (same \(p\), \(T\)) for the step from state 1 to state 2 in Fig. 5.2 and expect an increase \(\Delta S_{1-2} = \Delta_{mix} S\) larger zero.
We have
a closed system and thus can separate the identical ideal gases reversibly in a second step, i.e. \(\Delta
S_{1-3} = 0\). Now state 1 and state 3 are identical and since \(S\) is a state function \(dS\)
must be zero in disagreement to \(\Delta S_{1-2} \gt 0\).
As we will see in TdK II the solution to this paradox is that the above discussed classical approach only holds for distinguishable particles, however, identical particles can not be distinguished! A full understanding needs a quantum mechanical description of the particles and translates into a change in the phase space volume:
|
| \begin{equation*} \mbox{Distinguishable} \quad S \propto N \ln V \quad \rightarrow \quad S \propto N \ln \frac{V}{N} \quad \mbox{Indistinguishable} \label{eq:S_dis_indis} \end{equation*} | (5.7) |
© J. Carstensen (TD Kin I)