2.14
Calculation of \(\mu\) for different models
For virial coefficients as well as for the vdW-equation we will prove some quite fundamental
results related to the Joule-Thomson coefficient \(\mu\). The following equation already needs results from
the second law of thermodynamics, i.e. the entropy \(S\) is used as well as a Maxwell relation between
mixed partial derivatives. So you may take this section as a motivation for discussing the second law, because obviously
not all problems can be solved just by conservation of energy.
|
| | \begin{equation*} \begin{split}
\mu \;=&\quad \left(\frac{\partial T}{\partial p} \right)_H = - \frac{\left(\frac{\partial H}{\partial p} \right)_T}{\left(\frac{\partial
H}{\partial T} \right)_p} \stackrel{1}=\frac{T\,\left(\frac{\partial V}{\partial T} \right)_p-V}{C_p}\stackrel{2}=\frac{V}{C_p}\left(\alpha
T - 1 \right)\\ 1\;: \;& \quad - \left(\frac{\partial H}{\partial p} \right)_T = \left(\frac{\partial}{\partial p} \right)_T
\left(-T\,dS-V\,dp \right) = - T \left(\frac{\partial S}{\partial p} \right)_T - V = T\, \left(\frac{\partial V}{\partial
T} \right)_p -V\\ 2\;:\; & \quad \alpha = \frac{1}{V} \left(\frac{\partial V}{\partial T} \right)_p \quad \mbox{$\alpha$:
expansion coefficient.}\\ \end{split} \label{eq:mu_Joule-Thomson} \end{equation*} | (2.42) |
The expansion coefficient we discussed before in Eq. (2.6). Applying the above equation to different thermal equations of state we find
-
Ideal gas
|
| | \begin{equation*} \mu_{ideal} =
\frac{T\,\left(\frac{\partial V}{\partial T} \right)_p-V}{C_p} = \frac{1}{C_p}\left(\frac{n\,R\,T}{p} -V \right) = 0 \label{eq:mu_ideal}
\end{equation*} | (2.43) |
-
Virial approach according to the Berlin form (cf. Eq. (1.10)): \(V = R\,T/p + B\)
|
| | \begin{equation*} \mu_{virial}(T)
= \frac{T\,\left(\frac{\partial V}{\partial T} \right)_p-V}{C_p} = \frac{T\left(\frac{R}{p} + \left(\frac{\partial B}{\partial
T} \right)_p\right)-\left(\frac{R\,T}{p}+B\right)}{C_p} = \frac{T\left(\frac{\partial B}{\partial T}\right)_p-B}{C_p} \label{eq:mu_virial}
\end{equation*} | (2.44) |
At the Boyle temperature \(T_B\) the virial coefficient \(B\)
is zero (the gas behaves much like a perfect gas), thus \(\mu(T \rightarrow T_B) = 0\).
-
vdW approach with \(B = b - a / (RT)\) (cf. Eq. (1.25))
|
| | \begin{equation*} \mu_{vdW} = \frac{T\left(\frac{\partial
B}{\partial T}\right)_p-B}{C_p} = \frac{\frac{2\,a}{RT}-b}{C_p} \quad \Rightarrow \quad T_i = \frac{2\,a}{R\,b} \label{eq:mu_vdW}
\end{equation*} | (2.45) |
with \(T_i\): inversion temperature where \(\mu\) changes sign.
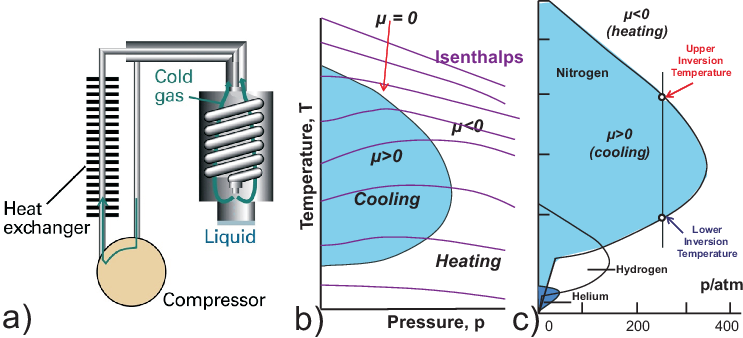
The Joule-Thomson effect is highly relevant for cooling. Fig. 2.10 shows the Linde process for production of liquid air: The gas must be beneath its inversion temperature
(examples for upper inversion temperatures: He: 35 K, H\(_2\): 224 K, N\(_2\): 866 K). The gas
cooled by expansion cools compressed gas, further expansion could lead to liquid.
© J. Carstensen (TD Kin I)