 |
Writing these modules, I found it surprisingly hard to find data or good metallographic
pictures for the plain carbon steel of the preceding chapter. |
|
 |
Well, there is a simple reason for that: There is practically no such thing as
plain carbon steel - and probably never has been. |
|
 |
Steel practically always contains other elements besides carbon too, which were
added intentionally or unintentionally. |
 |
Unintentional elements are in particular Sulfur (S) and Phosphorous (P);
but also Sn, As and Sb. |
|
 |
All these elements tend to diffuse to grain boundaries where they might segregate;
reducing the cohesive strength - the steel becomes brittle. If it does not happen right away, it might happen after some
temper treatment; as a result we have (undesired) temper brittleness. |
| | |
| |
|
 |
By now you should be sensitive to words like "tend" and "might",
which indicate that things are not so simple and easy. | |
|
|
 |
Phosphorous, for example, is not always harmful.
In properly treated steel, it might be beneficial, too, as shown below. |
|
|
 |
Since the "bog iron" (German: "Raseneisenerz"),
used for millennia to make iron and steel, contained relatively large amounts of P, it "might" have been
crucial for the early smiths to keep the Phosphorous from segregating to grain boundaries. What bog iron looks like is shown
on the right - we all have seen stones like that, but possibly not recognized what it was. |
|
|
 |
However, if you were lucky, some other elements contained in your
iron "might" have helped in this respect and you may never have noticed that you had a problem. |
|
 |
But generally, some elements, in particular Sulfur S (and P), are
almost always bad news, and not easy to avoid. |
|
|
 |
But fortunately, Manganese (Mn) is also quite ubiquitous - and it sort of "soaks
up" the Sulfur (by forming immobile sulfides). | |
|
 |
We thus have a first reason for adding something else:
To neutralize bad effects of unwanted, but hard to avoid trace impurities.
But this, while being quite important, is nevertheless only a minor point for making
alloyed steels, sort of a fringe benefit. |
|
|
 |
So small wonder that you will always find 0.5 % -1 %or so of Mn in practically
any alloy steel (and in "plain carbon", too). | |
| |
|
 |
The major reasons for adding all kinds of
elements to carbon steel are: |
|
 |
1. Improved strength while maintaining good ductility and in particular
workability ("Verarbeitbarkeit"). The key words in this context are solution
strengthening and precipitation
hardening ("Mischkristall-
und Ausscheidungshärtung") while maintaining weldability ("Schweißbarkeit"). |
|
 |
2. Improved hardenability.
The key is to enable martensite formation even at relatively low cooling rates
so that it can occur in the interior of massive steel pieces , too. In German, hardenability is called "Härtungstiefe"
(= "hardening depth"), which gives a better impression of what is meant: Even regions deep in the bulk, which
by necessity cool down more slowly than surface near region, become "hard", i.e. experience martensite
formation. |
|
 |
3. Improved corrosion resistance.
The key word is "stainless steel", resulting from rather large additions
of Chromium (Cr). |
|
 |
4. Stabilized austenite at low temperatures. In other words, we get (nonmagnetic)
austenitic
steel (with an fcc lattice) at room temperature (and somewhat below). It
is almost, but not quite the same thing as point 3. from above. |
 |
In addition, we should not forget that properties like weldability,
and pedestrian concerns like money, are also part of the alloying game |
 |
All the obviously desirable features from above can be achieved to some extent
by adding a suitable amount of the right elements. |
|
 |
To make things complicated, most elements do several things from the list above,
and a combination of two elements usually does not just produce the sum of the individual properties, but something new. |
|
 |
In addition, improving one property by adding a certain element might easily produce
problems with some other properties. You many have to compromise. |
|
 |
And not to forget: As we have seen by alloying Iron with just Carbon: Many variations
of properties are possible with just one element! |
|
 |
In discussing, not to mention making alloyed
steels, a certain amount of alchemy is in evidence, even today. And new discoveries and new steels will certainly come forth
in the future, too. |
|
 |
The link provides a short list
of some alloying elements and what they are used for. |
 |
It is entirely impossible to touch all bases here. Let's just give the four categories
from above a cursory glance and make a basic distinction at the beginning: |
|
 |
We distinguish between
- Low-alloy steels: We only add less than about 2 weight % of the major
alloying element(s) (and usually keep the carbon concentration low)
- High-alloy steels: We add a lot more than 2 weight % and possibly
as much as 20 weight %.
|
|
 |
In between is "medium-alloy",
but that already goes to far in this context. |
|
|
|
Improved Strength and Good Workability |
| |
|
 |
Here we are generally talking low-alloy steels,
in particular with a rather low carbon concentration. The general idea is to avoid martensite formation, which is bad for
welding and shaping, but still have good strength properties. |
|
 |
If you want to shape a piece of material
by any method (for car bodies you just press some sheet metal in a form), you must have some "workability"; in
other words, you need some plastic deformation, i.e. ductility. Think of pure martensite as being like glass, and you get
the idea. |
|
 |
Weldability is a particular important part of "workability";
another one would be "hot pressability" (Heißpreßbarkeit") or "drawability" ("Ausziehbarkeit;
Tiefziehbarkeit"). Just consider how you would make a car body, if those two properties are non-existent, and you have
a good idea of how important "workability" is for mass production! |
 |
We clearly need strength (= "hardness")
without martensite formation. |
|
 |
This leaves us with all the basic mechanisms discussed in chapter 8 for strengthening.
|
|
 |
We thus add suitable elements to obtain:
- Solution hardening. Except for nitrogen, which dissolves as an interstitial like
carbon, all other suitable elements will always be of the substitutional solid solution type.
- Precipitation hardening. Either by forming finely dispersed hard and small carbides of the alloying elements, or by influencing the cementite formation to occur in fine
particles, or by producing precipitates of compounds of the alloying elements (e.g. borides, or intermetallic phases), or
by all of the above.
- Grain size reduction. You may produce small grains (i.e. from a martensitic transformation),
and/or keep small grains small by keeping grain boundaries from moving (and thus grains from growing) by precipitating suitable
elements there (without making the grain boundary brittle, of course). This will always lead to hardening, too.
|
 |
It only remains to check the "easy" elements of the
periodic table under all kinds of conditions. Let's do that for solution hardening first. |
|
|
|
 |
What we find is that Carbon and Nitrogen have by far the biggest direct effect
on the yield strength (owing to their being interstitials), and that Phosphorous in solution
is very good, too (but, remember, very bad if segregated in grain boundaries). |
|
|
 |
Then we have Silicon (Si), Manganese (Mn) Titanium (Ti) and Copper (Cu,
not shown) and some others as still pretty good solution hardener. Cu, however, has drawbacks (including its prize),
and Si causes problems here (also it is much in use for other purposes). |
|
|
 |
This leaves Mn, Ti, and to some extent Ni and Vanadium (V) as
alloying elements (we also had Mn to neutralize spurious S, if you remember). |
|
|
 |
Complex - but not difficult. We had much the same picture
before for Copper. | |
|
 |
In essence, we understand that part of steel alloying. |
|
|
| |
|
 |
Precipitation hardening can be more efficient than solution hardening, and indeed,
very small amounts of Boron (B; 0.005 %), or about 0.1 % of Niobium (Nb) or Vandium (V)
will produce considerable increases in strength. |
|
 |
Always provided that the heat treatment was right, the grain size is small, and
so on and so forth. |
|
 |
Just on example: Niobiumcarbide particle of about 1 nm size will increase the yield
stress from about 20 MPa to 200 MPa, at a concentration of about 0.1 weight % Nb, while "huge"
particles with about 10 nm diameter have practically no effect anymore! |
|
 |
We understand that immediately, by looking at the mechanism
of precipitation hardening. If a 1 nm particle can stop a dislocation completely, a 10 nm particle can
do no more - but we have 1000 times fewer 10 nm particles at a fixed solute concentration. |
|
 |
We also understand why these micro-alloyed steels
are rather recent developments: Try to optimize such a steel if you don't know what happens, can't see your precipitates
anyway, and can't measure their size and other properties for some quantitative data. In other words, with no knowledge
about deformation and dislocations, just optical microscopes, and without the whole bag of microanalytical tools, you are
simple blind. The best you can do is go by trial and error following up some guesses. |
 |
Anyway, with some basic understanding and giving proper care to their needs, micro-alloyed
steels may have much better strengths than "mild" carbon steels, with all other properties (exept the prize) being
comparable. |
 |
To some extent, micro-alloyed steels are the steel industry's answer to the Al
car-body challenge from Audi, because they allow to maintain the easy manufacturability
and strength of a steel car body, while considerably reducing the weight (the sheet metal can be thinner). |
| |
 |
Of course, you may now ask yourself a simple question: |
|
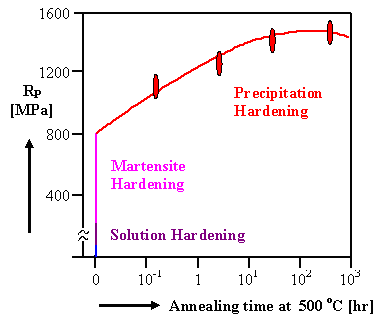 |
| Hardening mechanisms of maraging steel |
|
|
 |
Why do I always add carbon, if I can get
all kinds of hardening mechanisms from other elements, too? |
|
|
 |
Good thinking. Take carbon-free iron, add sizeable amounts of elements like Ni and
Co, and rather small amounts of, for example, Al, Si, Mo, or Ti. This gives you some
solution hardening if nothing else happens. | |
|
 |
Keep out P, S and so on, make sure the grain size is very small and the grain
boundaries not embrittled by segregation of the wrong elements. |
|
|
 |
Upon cooling down this alloy, some relatively soft martensite will form (No carbon!). This is when you shape your piece of steel in the form it is supposed to have when
it is finished. | |
|
 |
After that, you do some tempering, just right, to now form lots of very small intermetallic
precipitates between the major elements and the minor elements. |
|
|
| |
| |
 |
This puts some precipitation hardening on top of everything else and you end up
with "maraging steel" (short for martensitic aging), being fantastically strong
while still ductile - and being rather expensive. |
|
 |
The picture above shows the total effect with an increase of the tensile strength
to a fantastic 1500 MPa! Even larger values have been achieved while still keeping a maximum elongation of 6 %
- 8 % before fracture! |
|
 |
A maraging steel is what you use for landing gear of Jumbo jets, for ultra centrifuges
(needed for making atomic bombs) or for golf clubs (needed for hitting little balls). Interestingly, if you enter "maraging
steel" into Google, you will find either golf club advertisement, or stern warnings concerning trade restriction, but
very little useful information. We have a real high-tech material here! |
| |
|
|
Improved Hardenability
|
| |
|
 |
Shaping a sword, a car body, or whatever by banging, pressing, stamping, rolling
or drawing a piece of some rather soft steel into the desired shape, and then making it hard by heating and quenching, is
actually a great way of getting strong (= hard) products with comparably little effort. |
|
 |
So we want to keep this old-fashioned hardening method, known for millennia for
plain carbon steel, but we also want to make the result less sensitive to the cooling rate. Remember, with plain carbon
steel, you only get hard martensite in those parts of your work piece that cool down with cooling
rates of about 1000 K/s. |
|
 |
There is no way to achieve this kind of cooling rate with anything thicker than a few mm!
Therefore the only option left is to alloy the right elements to our plain carbon steel, hoping that this will lower the
austenite - martensite transformation temperature. This then might produce the good hardenability
we are after - which, remember, is not just a large hardness value, but hardness as deep
as possible into the bulk of a massive sample. |
|
 |
This brings us to an old
piece of wisdom concerning of what is better: Being practical, or being theoretical? If you don't have a good theory here, you do not even know if that feat is possible at all. Even if you trust your luck,
you have no idea of how much of what you should add. Good luck and all the time in the world to you practitioner! |
 |
Well, the truth is that we know a lot about alloying and hardenability, but we
really do not have a "final" theory yet, and a lot of what is known about hardenability did come from an empirically
established data base. |
|
 |
Thanks to Walter Jominy (the Chief
Metallurgist for Chrysler Corporation sometime before the war), there is at least a simple but accurate test to assess the
hardenability of a given sample. |
|
 |
Just take a standard size sample, heat it to some high temperature, and then spritz water
(at defined conditions, of course) at one end as shown below. The cooling rate will be different from one end to the other
of the sample, and all you do after it has cooled down completely, is to measure the hardness along its length. |
| |
|
 |
What you might find is shown to the right of the test set-up. |
|
 |
Plain carbon steel with sufficient carbon (e.g. 0.8 % ) may become very hard in the
region where the cooling rate was very high, but the bulk of the sample remains "soft" (red curve), while very
mild steel with little carbon (e.g. 0.3 %) just shows some hardening (green curve). |
|
 |
Now add some Cr, V, Mn, Ni, or Mo (or some other suitable
elements), and if you do everything right, you may obtain the blue curves - steels with good hardenability and adjustable
hardness. |
 |
All you have to do now is to check what happened to the other 10 or so
properties of supreme interest (ductility, weldabiliy, fracture toughness, corrosion resistance, ....). |
|
 |
If you are extremely lucky (and after 10 - 20 years of work), you may find a
new kind of steel with properties just right for your purpose and better than anything else available so far. |
| |
|
| |
Austenitic
and Stainless Steels |
| | | |
 |
We all know it: Iron and steel
rusts! What we probably do not know: Relatively pure iron ("wrought iron") rusts
far less then steel. |
| |
 |
In Delhi is a 1600 year old huge iron pillar (7 m tall, 6
tons in weight; see picture on the right) that does not rust. It was forged together
from many pieces of wrought iron with low carbon content. Its "secret" has recently been unraveled: The relatively
large amounts of P in the iron and in slag particles within the iron, catalyzed the formation of d-FeOOH
("Misawite") and a layer of crystalline phosphates that together form a stable protective layer. |
| | |
|
|
 |
In the "Württembergisches Landesmuseuum" (which we encounter in "sword" conncections, too) and in many others, iron bars
in the typical double-pyramid shape of the Celts as shown above are on display. Here si a picture: |
| | |
|
|
 |
If you ever visit these museeums (the display above is from the museeum in Heidelberg), don't
miss this part; you will experience some surprise: These wrought iron bars, about 2000 years old, look like new.
There is hardly a trace of rust. |
|
 |
But these are the exceptions to the rule: Iron and steel rusts! In the museeums mentioned
above, you can also see the evidence for this fact: Most steel objects like swords are just lumps of rust.
|
 |
In general, this is easy to understand: Since metals have too
many electrons by definition, and Oxygen has too few, metal-oxides will form in air. The noble metals are just the (rather
easy to understand) exception to the rule. |
|
 |
The oxidation of a metal exposed to air will go on as long as oxygen can meet metal, i.e.
as long as either one can diffuse through the oxide layers formed. |
|
 |
Stainless metals, obviously not decomposing into oxides
foreever, thus can only exist if the unavoidable oxide layer formed in air will be impenetrable to oxygen as soon as a certain
(small) thickness has been reached. |
|
 |
This is not all that difficult to achieve, after all, metals (and other reactive elements)
like Al, Si, Pb, Cu, Cr, ... are quite stable in air (at room temperature), and, as we
have seen, even some relatively pure iron does not rust. |
 |
Iron, plain carbon steel, and many alloy steels, however, do generally not form a stable oxide - they rust! And sooner or later our car body, sword, or cooking pot
is just a piece of ugly iron(hydro)oxide. |
|
 |
And there is nothing particular systematic that you can do. The method of choice, of course,
is to paint the object, or more generally, to apply a protective coating, e.g. paint,
Zn, Cd, or Cr, or if money is of no consequence, Au. But this will only help for some time if
the object is mechanically stressed (i.e. used) because than the thin protective layer
will sooner or later been worn off or develops cracks - rusting just starts later, as we all know. |
 |
The alternative is to alloy a sufficient amount
of typically Cr, so that the surface always is covered with a stable Cr2O3
layer. |
|
 |
The minimum amount of Cr you must add is 13 % (a number that can actually be
calculated), but up to 25 % or so are used. |
|
 |
But now you have high alloy steel; and while it may not
easily corrode, its properties may also be quite different from plain carbon steel. |
 |
Staying simple, you can get stainless steel by only alloying pure Fe (no
carbon) with Cr and nothing else. |
|
 |
But even then you will get something new: Fe - Cr alloys stay ferritic
(i.e.. in the bcc phase) at all temperatures - they do not form fcc austenite
at all. Well, no reason why they should, considering that this is no longer Fe with a little bit of something.
|
|
 |
The problem, however, is that now you have no possibility of using some kind of martensitic
transformation for hardening. |
 |
So if you also want strength, weldability and so on, you start a whole new game
of going through the periodic table in search of proper additional alloying elements. |
|
 |
Adding some Carbon again will help; 0.6 % is already enough to produce some martensite
and thus hardenability. Simple Fe - Cr - C stainless steels, quenched and tempered, are indeed used for, e.g., ball
bearings, kitchen knives or surgical instruments. |
|
 |
We now have stainless steel, with a bcc lattice at room temperature (lossely still
called "ferrite"), it is also "ferro"-magnetic (try your kitchen ware). But we can do more than that
with high alloy steel containing a lot of Cr. |
 |
Besides having sufficient Cr, add some Ni (say 10 %) and
the ubiquitious Manganese (about 1%). |
|
 |
What you will obtain is a steel that is still austenitic
at room temperature (i.e a fcc and non-magnetic)). It is not the
stable phase at room temperature, but the transformation temperature is lowered and never takes place for normal cooling
rates. |
|
 |
This is mainly a result of the Ni addition; the transformation temperature goes rapidly
down with increasing Ni concentration (from 914 oC at 0 % Ni to 720 oC
for 8% Ni, or to 600 oC for 15 % Ni. |
 |
Austenitic steels are materials quite different from regular steel.
|
|
 |
Not only are they stable in corrosive environments (thanks to stable Cr2O3
on their surface) and non-magnetic. |
|
 |
They are relatively tough but still more ductile than regular steel and thus are easy to work
with because they can be pressed or drawn. They also have better creep properties (we will learn what that is in chapter
10) A certain problem is that they work harden very rapidly, which makes them difficult to machine. |
|
 |
You will find a lot of austenitic steel around you. Your kitchen sink will almost certainly
consist of austenitic steel, but also the inside of your electron microscope, and much piping in your nuclear power plant. |
 |
Well, so much for steel in just two short chapters. Three final remarks are in
order: |
|
 |
1. I found it is impossible to do "steel" justice without including some of its history.
|
|
 |
2. It is even more impossible (excuse the oxymoron), to do steel justice without having
gone through the "basics" first, as put down in chapters 2 to 7. |
|
 |
3. There is much more scientific stuff around steel (and any other alloy) that was
carefully avoided here in order not produce system crash at this level. In particular there are things like TTT-diagrams
("temperature-time-transformation"), semiquantitative complicated diagrams that reveal , e.g., what will happen
to a piece of alloy if it is cooled down with a specific cooling rate. |
 |
I hope that you understood the basic messages: |
|
 |
1. Steel is just a collective name for an incredibly complicated set of materials with
wildly different properties. |
|
 |
2. But everything can be understood in principle
by understanding the atomic mechanisms of "strength" (and "fracture"). There is no
mystery anymore, and no magic is needed to produce a wide variety of products reliably. |
|
 |
3. We are just in the transition period where development of new steel alloys (of Iron
and other metals) switches from the (highly educated) "trial and error" method, to a development that is guided
by scientific principles based on the theory of the atomic structure of the material. |
© H. Föll (MaWi 1 Skript)
