|
So far we have looked at how light interacts with matter; eventually disappearing
by some kind of absorption. Here we look - very briefly - at the principles of generating
light. |
|
 |
If we were to look at all the electromagnetic radiation there is - from long
wave radio to g rays - we would now have to start a long lecture course by itself. Even
if we restrict ourselves to visible light plus a bit of infrared and ultraviolet, we
still have a large task ahead of us. |
|
 |
In the context of this lecture course we can do no more than to enumerate major light generating
principles together with a few key properties. |
 |
Any light source will be characterized by the kind of light it produces. For that
we look first at the properties of the light produced : |
| |
- Monochromatic or polychromatic. In
the latter case we want to know the spectrum. The link
gives an example, it shows the spectrum of our most important light source.
- Spectral details. Even for monochromatic light of wavelength l we need to know
details like the spread ±Dl and the stability in time, i.e. (t).
- Emission characteristic. Is the light emitted in just one direction
(as in a Laser beam), in all directions evenly, or with some angular characteristics?
- Polarization. Is the light polarized linearly, circular, elliptical or not at all
(meaning that all polarization directions occur with the same probability).
- Intensity or energy density. Possibly as a function of l,
emittance angle, polarization and so on.
|
 |
Being technically minded we are just as interested in technical properties: |
|
|
- Power
efficiency
hlight, telling us how many percent of the energy flowing into a light source
comes out as energy of light we want.
- Luminous
efficacy, telling us how the eye perceives efficiency. In other words, if a green and
violet light source have the same efficiency and produce the same number of photons per second that are entering your eye,
the green one will have a far higher efficacy, appearing brighter, because the eye is
far more sensitive to green than to violet light.
- Device lifetime. After how many hours of operation do you have to replace
your light source by a new one?
- Maintenance. Is some regular service needed including, e.g., replacements
of parts or re-calibration?
- Costs. How much do you have to pay for the device up front? How large are
the operating costs?
|
|
 |
The technical properties are the more interesting ones for everyday life. For very basic research
it might be the other way around. |
 |
Roughly
20 % of the electrical energy produced on the planet goes into light production. This is more electricity than all
nuclear and water power plants produce. |
|
 |
The CO2 produced just for illumination is about 70 % of that from
cars and three times more than that from air traffic. The picture gives an idea of what that implies. |
| |
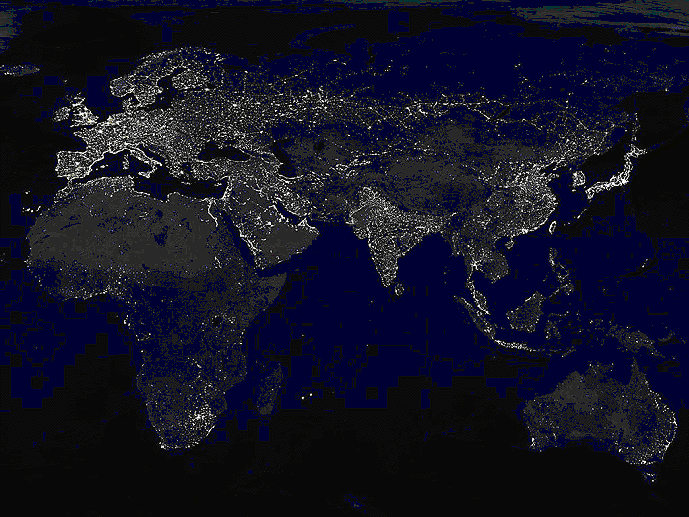 |
| The planet at night showing artificial illumination. |
|
 |
Given
the energy and climate crisis, the need for new light sources with high efficiency / efficacy is obvious. |
|
 |
Helping to save the planet in this way is one of the major jobs for Material Scientists and
Engineers. Right now, and for many years to come. |
|
| |
 |
Anything very hot emits light and if the "anything" is a "black-body"
we know the spectrum emitted as a function of temperature because this is is given Planck's
famous equation |
|
|
En · dn
| = | | |
|
8 · p · n3 · (hn)2
h3 · c3 · kT |
· d(hn) |
| | |
| For an elegant derivation see this link |
|
 |
|
|
 |
Light generated by hot bodies we call "black
body radiation" or incandescend light, resulting from incandescence |
|
 |
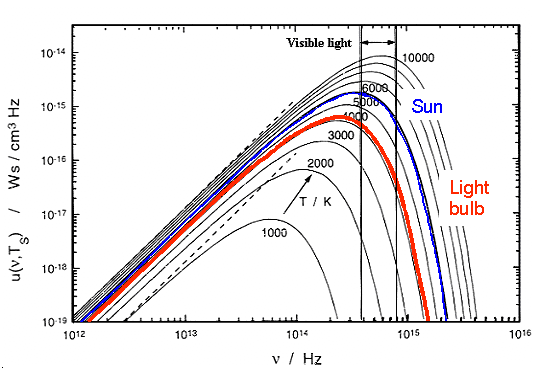
The temperature of the sun surface is about 5800 K. Tungsten (W), the typical
filament material of a light bulb, melts at 3683 K. The temperature of the light emitting part of a light bulb is
thus around 3000 K. If you look at the spectrum above, one conclusion is inevitable: |
| |
| Black body radiation light sources will always have a lousy efficiency |
|
|
 |
Most of the radiation emitted is not in the frequency range of interest, and there is little
you can do. Moreover, quite a bit of the energy input is wasted by simply heating the device. Ideal black body radiators
at 4000 K or 7000 K have efficiencies around 7 % or 14 %, respectively. Our ubiquitous light
bulb converts the electrical power UI flowing through with an efficiency of about 5 % to light energy.
|
|
 |
Since a lot of the electrical energy produced goes into light, and given the current and future
energy crisis, this needs to be changed presto. There is thus no choice but to employ another principle for producing light |
| | |
|
 |
Hot bodies emit light because thermal energy is sufficient to move electrons up
to all kinds of high energy levels Ehigh from which they transit to all kinds of lower lying levels
Elow. Since all kinds of Ehigh – Elow
occur, the spectrum emitted covers a large wavelength region. |
|
 |
If we want to make light more efficiently we have to make sure that DE = hn's available for electrons are in the visible range. Do
we have examples for that? |
|
 |
Words like "Luminescence" or "Phosphorescence"
come to mind. Certain materials (called "phosphors" on occasion)
have convenient energy levels for visible light production and as soon as you feed them "somehow" with energy
so their electrons can populate the upper energy levels, they start to luminesce or
phosphoresce. The difference between those modes is simple: |
| |
| Luminescence: | |
General name for "cold" light production |
| | |
|
| Fluorescence: | |
Light production shortly after energy input
Short life time of excited level (<
µs) | | | |
| | Phosphorescence: |
|
Light production long after energy input
Long life time of excited level (> ms) |
|
|
 |
So what we want is fluorescence. The big question
now is how we "excite" the luminescent material. In other words, how do we supply the energy flow necessary for
kicking those electrons up to the proper energy levels all the time. Let's enumerate the major
possibilities. A more complete list can be found via the link |
| | |
- Cathodoluminescence refers to the use of "cathode rays" or simple
electrons with sufficient energy. The light generated by good old TV tubes (before the advent of flat panel displays) is
generated by cathodoluminescence and so is the light from "fluorescent tubes".
- Electroluminescence refers to excitation by simply running a current through
the system that neither heats nor generates a plasma but moves carriers to the high energy level called conduction
band in this case.
- Photoluminescence refers to excitation by light of a somewhat higher energy than
what we want to generate. Seems to defy the purpose but is nonetheless an important mechanism as we shall see.
|
| |
 |
This link lists about 10 more types
of luminescence; some with quite interesting properties. |
 |
Cathodoluminescence is the principle
behind what we generally know as fluorescent lamp or fluorescent
tube. We have a gas-discharge lamp and the electrons in the plasma have enough energy to excite the mercury vapor
in the plasma produced. The excited mercury atoms then produce ultraviolet light that then causes a phosphor to fluoresce,
producing visible light. So we do have indirectly photoluminescence in there as well. |
|
 |
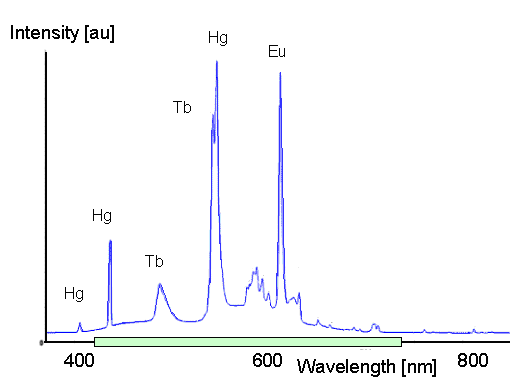
The major advantage is a good efficiency around 20 %. A typical spectrum is shown below,
the green bar marks the visible region. |
|
|
|
|
 |
The picture makes clear why luminescence can have a high efficiency: A lot of the energy going
in comes out as light with sharp frequencies in the visible range. There is no need to always produce a lot of infrared
and ultraviolet light in the process. |
|
 |
The disadvantages are clear, too. Mercury (Hg) is needed, causing environmental hazards,
and the rare earths Terbium (Tb) and Europium (Eu) are called "rare" for a reason. Right now (2011)
China controls around 90 % of the rare earth market and what that means is that prices are bound to go up in years
to come. You also have to condier that teh ligh tmay appear white because it haas trhe right moc of wavelnegth but that
its spectrum is rather different form that of the sun or any other black body radiator. |
|
 |
Using direct photoluminescence as light source is of course
(?) ridiculous so we won't discuss it any more. |
 |
This leaves us with electroluminescence
or, to use another word for essentially the same thing, radiant electron - hole recombination
in semiconductors. In yet other words: I'm now turning to LEDs as light sources. |
|
 |
This can be done with very large efficiencies. We are talking the future of lighting here. |
|
 |
How it's done we will see briefly in chapter 5.3.
Otherwise use these links |
| |
|
© H. Föll (Advanced Materials B, part 1 - script)