|
In alphabetical order we have: |
| | |
 |
Bioluminescence, i.e. luminescence generated by a living organism.
|
|
 |
The light generating molecules become excited by a chemical reaction; bioluminescence
is thus related to chemiluminescence. Some biological entities like bugs, e.g. fireflies,
denizens of the deep like anglerfish, some mushrooms and even bacteria can control this reaction. They produce the chemicals
luciferin (a pigment) and luciferase (an enzyme).
The luciferin reacts with oxygen to create light. The luciferase acts as a catalyst to speed up the reaction. |
| |
 |
 |
Firefly attracting females with lit up behind
(Source Wikipedia) |
Mushroom; glowing just for fun?
(Source amazingdata.com) |
|
 |
Chemiluminescence (or "chemoluminescence")
results from some (actually amazingly few) chemical or electrochemical reactions. |
|
 |
The energy needed comes from the reaction enthalpy. The reaction produces some new molecule
that can have its electrons in an exited state right after it was formed. Decay to a ground state may then produce visible
light (the exception) or release the energy in some other way (the rule). |
|
 |
The flash of light you see when some dynamite explodes is not chemiluminescence even so the
energy comes from a chemical reaction but simply black
body radiation or incandescence from things getting very hot very quickly. |
 |
Crystalloluminescence is occasionally
produced during crystallization |
|
 |
It's another variant of chemiluminescence because the energy comes essentially from bonding
between atoms. |
 |
Electroluminescence generates light in
response to an electric current passing through some material |
|
 |
In essence, electroluminescence results radiative recombination of electrons and holes; typically
(but not exclusively) in semiconductors. We have treated
that extensively before. It is the basis for LED's and semiconductor Lasers and thus of prime importance in the context
of light sources. |
|
 |
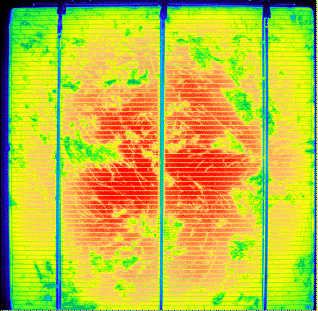
Recently electroluminescence is also used for characterizing solar cell. Feed a large current
into a good solar cell in the dark and your IR camera will see (weak) electroluminescence
even so Si is an indirect semiconductor. The reason is simple: Good solar cells, by definition, are Si devices
where all regular recombination
channels have been "closed off", i.e. made inefficient, otherwise the solar cell cannot be good. Radiant recombination
then "wins" and is raised to a level where it can be detected The following picture shows an example. |
| |
|
 |
Cathodoluminescence occurs when an electron
beam impacts on a luminescent material such as a "phosphor" |
|
 |
For almost 100 years cathodoluminescence was used for making displays, your classical
cathode ray tube
or TV tube that was recently replaced by flat screen displays like LCD's. |
|
 |
We still need cathodoluminescence for electron microscope (TEM type) screens
for obvious reasons. But we can also look at the cathodoluminescence that specimen produce in the electron beam of an SEM; then we use it as an analytical tool. The "phosphors" tend to be large bandgap
semiconductors line ZnO, again for (hopefully) obvious reasons; if color is needed like for an (old fashioned) TV,
more involved different materials emitting red, green, and blue are necessary. |
 |
Mechanoluminescence, resulting from any
mechanical action on a solid, can be subdivided into: |
|
 |
Triboluminescence (one of my favorites). Take a lump
of sugar, go into a dark room, wait a while so your eyes adapt, and than violently crush that sugar between your teeth (keeping
your lips open, so you can see your teeth and the sugar in a mirror). Blue flashes of light will be generated! Triboluminescence
happens quite a lot, it is just not seen at daylight conditions because it is typically weak. It happens when bonds in a
material are broken because that material is scratched, crushed, or rubbed. The effect is not really understood; separation
and reunification of electrical charges seems to play a role; and there might simply be sparking in large electric fields.
|
|
 |
Fractoluminescence, pretty much the same thing as
triboluminescence. |
|
 |
Piezoluminescence, is produced by the action of pressure
on, well, piezoelectric materials. It's different from the above because you do not need to break bonds but just some elastic
deformation. |
 |
Photoluminescence is caused by moving electrons
to energetically higher levels through the absorption of photons. |
|
 |
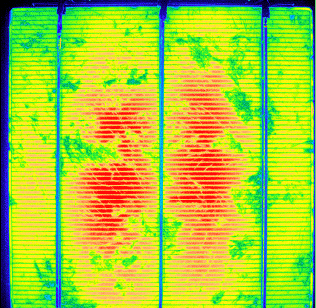
It's easily done in semiconductors with photons of energy larger than the bandgap, radiating
recombination channels than produce bandgap light. The solar cell picture above gives an example. It was irradiated with
very intense red light; the luminescence occurs in the IR. |
 |
Radioluminescence is generated when some
materials are exposed to ionizing radiation like a, b
or g rays. |
|
 |
It was used, even so that is hard to believe nowadays, to make watch dials glow in the around
1960. A mixture of radium and copper-doped zinc sulfide was used to paint the dials, giving a greenish glow. Radioluminescence
is also used for detecting ionizing radiation, especially g rays, by analyzing the light
flashed generated when a g quant is absorbed in certain crystals. |
 |
Sonoluminescence is the emission of short
bursts of light from imploding bubbles in a liquid when excited by sound. |
|
 |
It had been known for some time but produced a lot of excitement in 1989 when stable single-bubbles
could be produced that emit intense light in very short bursts. The mechanism is not really clear at present and rather
outlandish explanations (e.g. an analogy to radiation from black holes or nuclear fusion taking place)) have been proposed
by well know scientists. It appears likely, however, that the bubbles "just" produce an extremely hot plasma (up
to 20.000 K) for very short times. |
 |
Thermoluminescence, describes the phenomenon
that certain crystalline materials emit light when heated that is not black body radiation
or incandescence |
|
 |
What happens is that previously absorbed energy from, e.g., electromagnetic or ionizing radiation
was stored (meaning the excited electrons just stay on upper energy levels), typically at defects. It is released in the
form of light if some thermal energy allows the excited electrons to overcome the energy barrier that kept then "up". |
|
 |
Thermoluminescence is an important method for dating some archeological artifacts. Ceramic
parts being buried receive some ionizing dose from radioactive elements in the soil or from cosmic rays that is proportional
to their age - and so is the intensity of the luminescence produced upon heating. |
| |
|
© H. Föll (Advanced Materials B, part 1 - script)