5.9
Phase separation
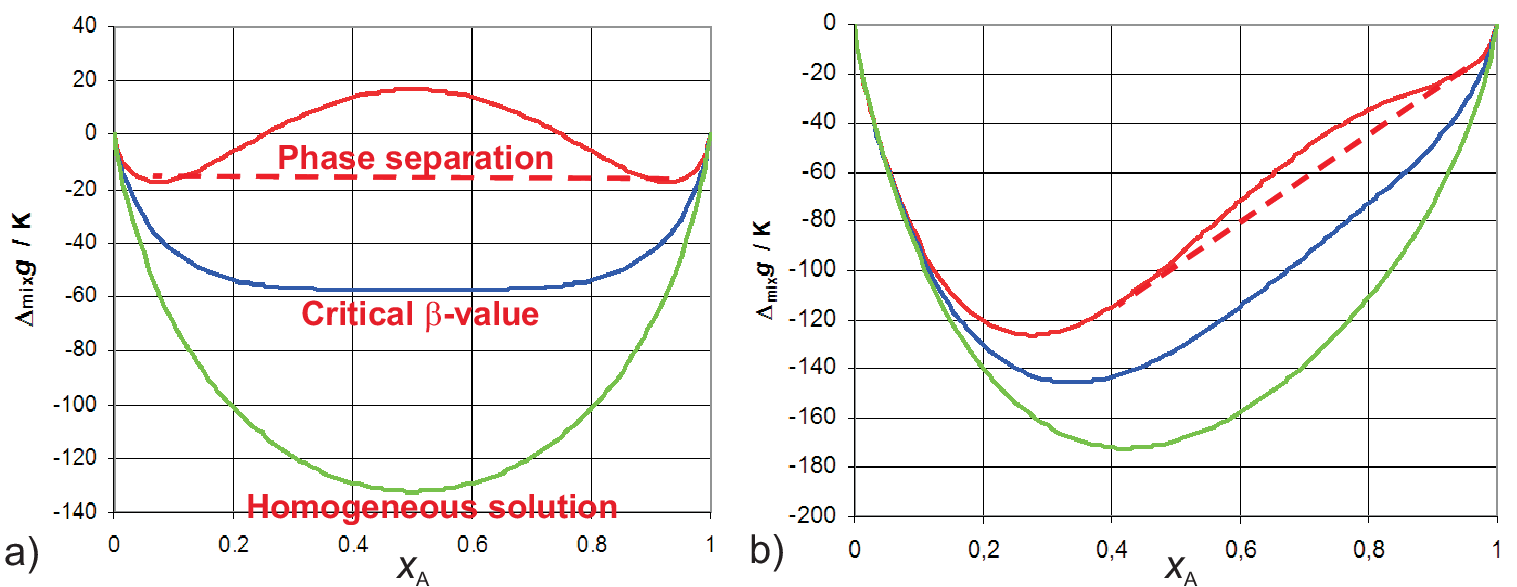
For the regular solution (cf. Eq. (5.26)) as well as for the asymmetric interaction enthalpy according to Eq. (5.28) graphs for \(\Delta_{mix} G_m\) for different values of \(\beta\) are shown
in Fig. 5.6. For large values of
\(\beta\) a concave branch in the molar excess Gibbs potential is found. Such parts are not stable (cannot
exist) and must be replaced by a common tangent as illustrated by the dashed lines. Physically this implies a phase separation
between the two compositions connected by the common tangent. For regular solutions the two connected points are arranged
symmetrically around \(x_A = 0.5\) while for the asymmetric enthalpy both points are arranged asymmetrically.
Next we will calculate for regular solutions the compositions between
which phase separation exists. As visible in Fig. 5.6 these points are found at the minima of \(\Delta_{mix} G_m\), i.e.
|
| | \begin{equation*} \begin{split}
0 &=\;\; \frac{\partial \Delta_{mix} G_m}{\partial x_A} \\ &=\;\; R\,T\, \frac{x_A \ln x_A + (1-x_A) \ln (1- x_A) + \beta
\, x_A \,(1-x_A) }{\partial x_A}\\ \Rightarrow & \quad 0 =\, \ln\frac{x_A}{1-x_A} + \beta \left(1 - 2 \,x_A \right) \\ \end{split}
\label{eq:dG_ex_zero} \end{equation*} | (5.29) |
This is a transcendental equation for which the solutions can only be found numerically. Fig. 5.7 shows the typical shape of the boundary curve vs. \(\beta\). As indicated above \(\beta \propto 1/T\) so with increasing \(T\) the dimensionless
scaling factor \(\beta\) decreases. For \(\beta \lt 2\) phase separation does not exist anymore,
i.e. it vanishes at high temperature. So \(\beta = 2\) is the critical value which is indicated in Fig. 5.6 a) as well. So phase separation exists
only for repulsive forces A-B between the molecules. Phase separation is the response of the system to two opposing effects:
the increase of entropy which always favors mixing and the increase of enthalpy due to the repulsive forces which supports
demixing.
© J. Carstensen (TD Kin I)