The concept of excess functions is similar to that of residual functions (cf. section 3.28), however, the excess functions are restricted to mixing. Both represent the difference between a real and an ideal state function. For the real as well as for the ideal system the sum of the separated pure components is subtracted to calculate the \(\Delta_{mix}\) parameter, i.e.
Link to caloric data:
\(\Omega, \,\beta \lt 0\): mixing is exothermic (heat release), \(\left|U_{A-B}\right| \gt \left|U_{A-A}\right|,\; \left|U_{B-B}\right|\), e.g. chloroform, acetone.
\(\Omega, \,\beta \gt 0\): mixing is endothermic (heat uptake), \(\left|U_{A-B}\right| \lt \left|U_{A-A}\right|,\; \left|U_{B-B}\right|\), e.g. aqueous solutions of hydrophobic compounds.
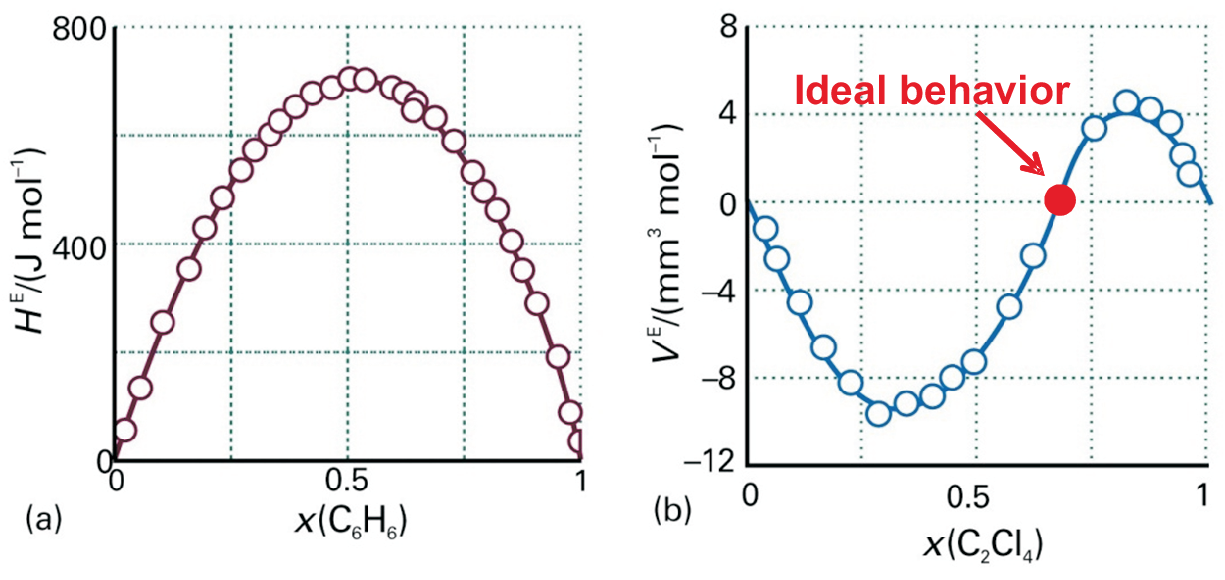
Normal regular solution: \(G^{ex} = H^{ex}\) are symmetric to \(x = 0.5\). An example for such a symmetric excess enthalpy is shown in Fig. 5.5 a) for mixing of tetrachloroethene and cyclopentane.
As an example for a non-regular solution the asymmetric excess volume for mixing of tetrachloroethene and cyclopentane is shown in Fig. 5.5 b).

Such an asymmetry as shown for the excess volume in Fig. 5.5 b) needs an asymmetric interaction between particles A and B which can mathematically be implemented for the excess enthalpy most easily as
© J. Carstensen (TD Kin I)