|
Deposition of a thin layer must start with a "clean" substrate surface
on which the first atomic / molecular layer of the film must nucleate. |
|
|
 |
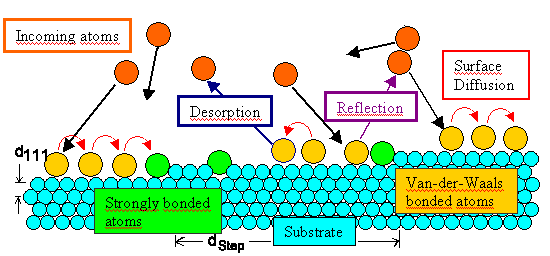
There are many possible interactions between the substrate and "first"
incoming atoms. | |
|
 |
As the interaction energy goes up we move from "some" absorption to
physisorption (secondary bonds are formed) to chemisorption (full bonding) |
|
|
 |
The sticking coefficient is a measure of the likelihood to find an incoming atom in the thin
film forming. | |
|
 |
Immobilization by some bonding is more likely at defects (= more partners). The initial stage
of nucleation is thus very defect sensitive. | |
| | |
| |
 |
Simple surface steps qualify as efficient "defects" for nucleation. |
|
|
|
 |
Small deviations from perfect orientation provide large step densities. Nucleation therefore
can be very sensitive to the precise {hkl} of the surface |
|
|
 |
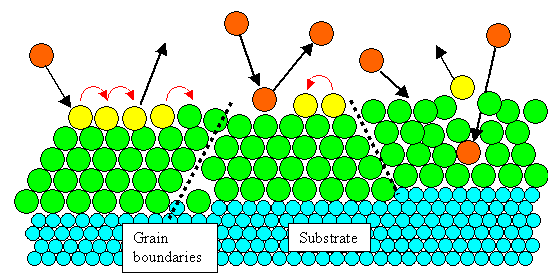
Intersections of (screw) dislocation lines with the surface also provide steps. |
|
|
 |
This may cause grain boundaries and other defects in the growing layer. |
|
|
 |
Scanning probe microscopy gives the experimental background |
|
| |
| |
| |
 |
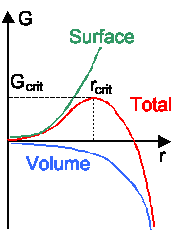
There is always a nucleation barrier that has to be overcome for the first B-clusters"
to form on A | |
|
|
 |
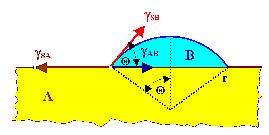
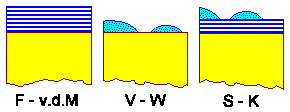
the three involved interface energies, all expressed in the "wetting angle", plus
possibly some strain are the decisive inputs for the resulting growth mode.
- Frank - van der Merve: Smooth layer-by-layer growth
- Vollmer - Weber: Island growth
- Stranski - Krastanov: Layer plus island growth
|
|
|
|
| |
|
|
|
© H. Föll (Semiconductor Technology - Script)