|
So you are an almost perfect Si crystal, or any
other crystal, for that matter. The "almost" refers to the fact that you have a surface somewhere, which qualifies
as a defect among purists. |
|  |
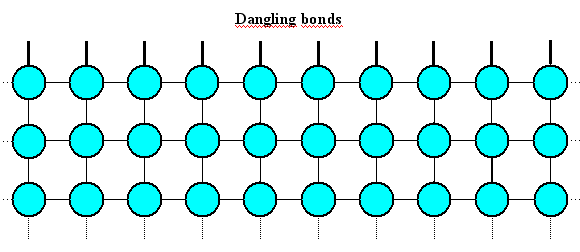
This is painful because your surface atoms cannot bond to other atoms as they would like to
do; in stark contrast to your atoms in the bulk. That's why that surface has a certain amount of surface energy, coming
straight from the unsatisfied bonds of the atoms at the surface, which we will now call "dangling
bonds". This situation is shown in the first picture below. |
|
 |
Can you do something about about your surface that lowers the energy (actually the free enthalpy, but entropy is not all that important here)? Of course
you can - just get dirty. Oxidize, add a layer of water molecules, take whatever you find in the atmosphere and that forms
a bond with your atoms, gaining some energy in the process. That you and pretty much any other crystal will do this is proven
by the fact that most materials in air actually are covered with a thin layer of something. |
|  |
If you have been out in single confinement in a vacuum chamber, grab any molecule coming by
on occasion. You then reduce the number of molecules in the vacuum and lower the pressure, and people may abuse you by actually
taking you as a gettering layer in order to improve their vacuum (that's how, for example,
"ion getter pumps" work!). |
|
|
|
 |
However, people being only human after all, on occasion like to see things stark naked and
thus might strip you of all your nice surface layers and then put you into ultra high vacuum, where nothing fashionable
comes by for a long time. What are you going to do now? |
|  |
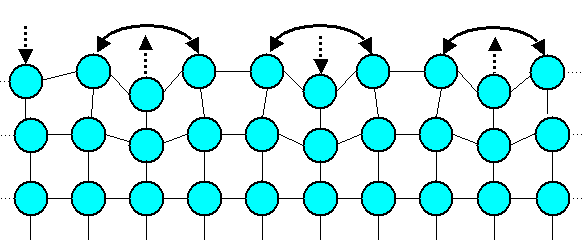
You use an old trick of thermodynamics: You spend some (elastic) energy first that allows
you to rearrange the outermost atom layers a bit in such a way that they can now form some additional bonds with their neighbors.
Maybe not very good bonds, but one has to take what one can get. |
|
 |
One possible way of doing that is shown in the second picture. Note that you can't do that
in one dimension only. In the picture the surface bonds with just one arrowhead must be perceived as coming out of the screen. |
 |
Is the way this has been drawn the way it will actually happen? Most certainly not! There are,
if you think about it, innumerable ways of achieving such a surface reconstruction,
and only one variant can be the best in terms of energy gain. |
|  |
It is quite unlikely that a Professor, being only human after all too, will hit on the best
way by just fooling around with balls and sticks. |
|
 |
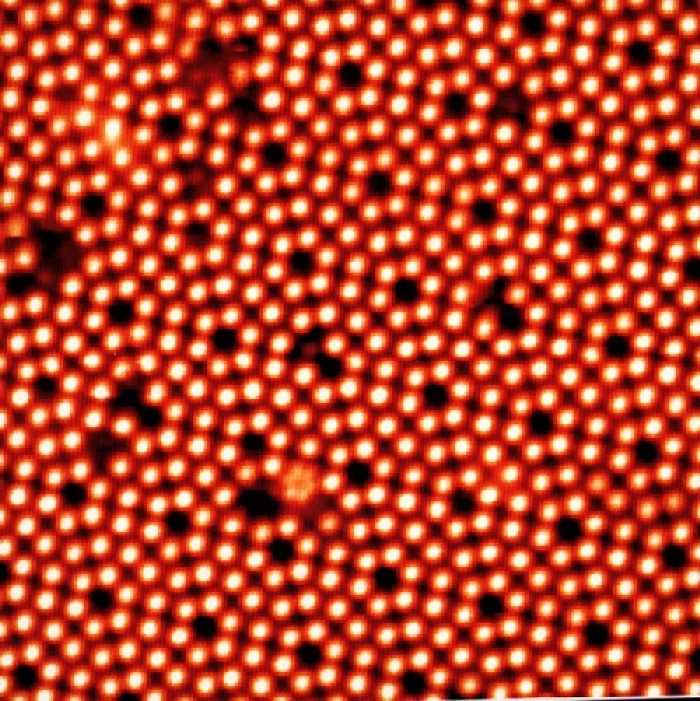
You, however, being a smart crystal, will automatically do the right thing and reconstruct
your surface in such a ways that the surface energy is minimized. And that can become quite complicated as an old friend, the reconstructed {111} Si surface, proves: |
| |
|
 |
What we learn from this are two points: |
|
 |
1. If you really have a clean surface of a crystal as a substrate for your thin film
deposition, chances are that it is reconstructed. This will, of course, influence to some degree what will happen in the beginning. |
|
 |
2. It is pretty hard to know (from theory) if a given surface reconstructs, and if
it does, what it will look like. |
| |
|
© H. Föll (Semiconductor Technology - Script)