 |
| Cis- (and Trans) Configuration | |||
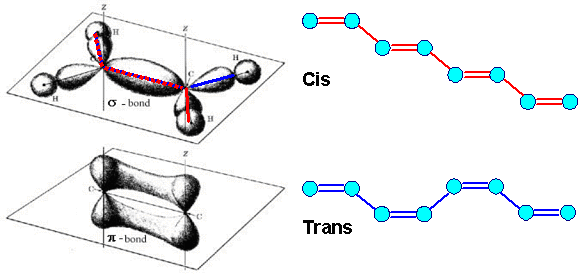
| Given the geometry of the sp2 hybridization required for every carbon atom of a conjugated polymer chain, the arrangement of carbon atoms can follow two basically different "recipes" as shown below | |||
| |||
| The meaning of "cis" and "trans" configuration becomes clear, but bear in mind that we also could have just a statistical arangement or some trans in mostly cis, or... . You get the point. | |||
|
| |||
| Conjugated polymer | ||
| Any polymer with alternating single- and double bonds along the chain | ||
| The general way of drawing that is —C==C—C==C—C==C—; of course always with one "something attached to every C-atom | ||
| However, looking at the three-dimensional bonding structure following from the sp2-hybrid orbitals of the carbon atom, it becomes clear that the chain cannot be perfectly linear. This allows then for variants for the same basic chemistry known as "cis" and "trans" configuaion | ||
| Peierls instability | ||
| See the extra module for that | ||
| Pi (p) - Bonds | |
| Sigma (s) - Bonds | |
| Trans- (and Cis) Configuration | |||
| Given the geometry of the sp2 hybridization required for every carbon atom of a conjugated polymer chain, the arrangement of carbon atoms can follow two basically different "recipes" as shown below | |||
| |||
| The meaning of "cis" and "trans" configuration becomes clear, but bear in mind that we also could have just a statistical arangement or some trans in mostly cis, or... . You get the point. | |||
© H. Föll (Semiconductors - Script)