|
|
| |
The Idea |
 |
The technical use of the term "gettering" (derived from "getting")
goes back to the good old time of vacuum tubes that enabled early electronics, in particular radio and TV. Parts of the
inside of the glass tubes was coated with a "getter" (typically titanium) that "got" the remaining gas
molecules by reacting with them, thus improving and maintaining vacuum conditions. |
|
 |
Just for the hell of it, here are two old electron tubes with their getter layer.
The big one is from around 1930, I guess, the smaller one might date to 1950/60. A decent radio needed 5 - 10 of those things;
your i-phone would need about 100.000.000.000 or so (plus a major power plant to supply the needed energy). Aren't you glad
we invented integrated circuits? |
| |
| |
| |
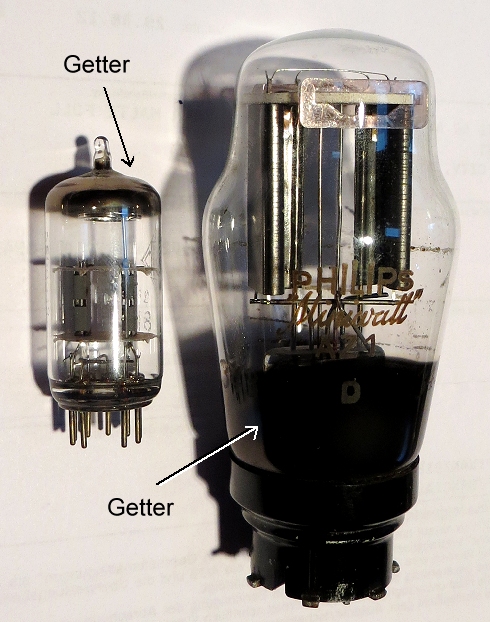 |
Electron tubes. One of those beauties is able to do essentially
what one transistor does nowadays. |
|
| |
| |
|
 |
While this kind of gettering is still used in vacuum technology, gettering
in silicon is somewhat different. The idea is to "get" or getter itinerant metal atoms that are still
present in the silicon crystal - albeit at very low concentrations. We want to imprison these metal atoms somewhere in the
bulk of the crystal and thus keep them from wreaking havoc in the active layer close to the surface that contains the transistors
or other electronic components. All one needs to know about the "wreaking havoc" part is that many metals (in
particular our friend, the iron) are able to kill transistors at concentrations in the ppqt
region, a concentration a billion times smaller than what chemists would call "high purity". |
|
 |
What could we use as "getter"? One might put some titanium, or some other highly
reactive stuff on the Si wafer backside, hoping
that they catch or getter impurity atoms as soon as they happen to come by. Backside or "external"
gettering is actually done to some extent, especially in solar cell technology, but in different ways. |
 |
Internal or intrinsic gettering, however,
is far more effective. The getter in this case consists of intrinsic defects that are distributed all over the bulk of the
silicon wafer - but not in the first (10 - 20) µm below the surface. This is important because these getter defects
would definitely kill transistors and thus must be avoided in the active region. |
|
 |
What are those intrinsic defects? They are oxygen
precipitates plus, perhaps, some dislocations created by these precipitates in their immediate environment. Here
is what this might look like: |
| |
| |
| |
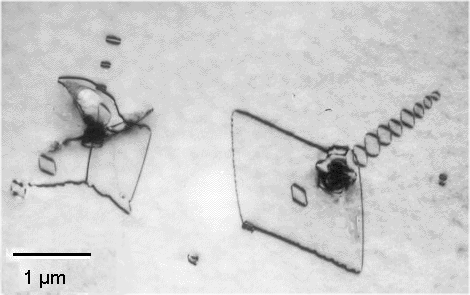 |
SiO2 precipitates plus some dislocations as seen in TEM.
You might have seen this picture before. |
| Source: My friends H. Strunk and B.O. Kolbesen |
|
| |
| |
|
 |
Defects like this generally attract point defects and incorporate them into their structure,
effectively immobilizing them. |
 |
We now need to answer two questions:
- How do we make those defects? Keeping in mind that we need just the right size
- neither too small nor too big.
- How can we produce a "denuded zone", a region free of those defects below the surface of the wafer?
If you made it through the nucleation modules and the Ostwald ripening module, you can come up with the answers:
- If you want to make oxygen precipitates, you must supply oxygen in supersaturation. If you want to make these precipitates
in a certain size and density, you must carefully determine the proper combination of initial oxygen concentration in your
crystal / wafer and the temperature treatments needed.
- If you want to avoid oxygen precipitation in surface near regions, you must decrease the oxygen concentration in these
regions to values low enough to prevent nucleation.
|
 |
This is clear enough. Armed with the phase diagram of silicon - oxygen, the diffusivity
of oxygen (and some other entities) in silicon, and some idea about nucleation, a material scientist
could now calculate the optimal strategy and tell you what kind of crystal you need to buy and what you must do with it.
|
|
 |
Ha ha! You, being the smith
engineer, do not live in the ideal world of science but in the real world, where money
is of importance. You know a few things about making microchips that can be sold because they are not prohibitively expensive:
- You cannot order Si wafers with any oxygen concentration but must go with what is available at reasonable cost. That
means you must live with oxygen concentrations somewhere in the 10 ppm range.
- You cannot go for some very special temperature treatment just for making oxygen precipitates because making transistors
already defines very special temperature treatments anyway, and these you simply have to do. The best thing would be if
those treatments would, as a beneficial side effect, produce the required oxygen precipitates, too.
- If anything comes on top of that, it must be affordable. You cannot, for example, afford very long tempering processes
if you need to process a couple of thousand wafers every day.
|
| | |
|
| |
Doing It |
 |
Now we are talking about extensive and expensive R&D efforts that will come
up with a process that is compatible with these requirements and then is rather specific to the company that developed it.
Since intrinsic gettering is important, it comes as no surprise that specific data are quite confidential.
Some general
data are known, however, and I will give you a taste treat of what is going on in what follows. |
|
 |
1.
Oxygen concentration.
You specify, for example, an oxygen concentration of 8.5 · 1017 cm–3
± some tolerance to your wafer supplier. Larger tolerance bandwidths make the wafers cheaper. |
|
 |
2.
Denuded zone formation
Nothing helps. You must, as a first step, keep your wafers at some rather high temperature
(e.g. 1100 oC; 2012 oF) for some time (e.g. 10 h). Oxygen atoms than are mobile and their diffusion length for these data is roughly around
10 µm. If you provide some environment that carries off any oxygen atoms that happens to come to the surface, the oxygen
concentration close to the surface will decrease to about 3 · 1017 cm–3
. This is not unlike the de-carburization of steel that might happen when you forge at high temperatures.
If you
increase the temperature, this will happen faster. Since time is money you tend to do that. However, your wafers are inside
a ultrapure quartz tube and above 1100 oC this (expensive) tube starts to soften and its lifetime decreases.
In other words: you must find a compromise. |
|
 |
3.
Nucleation of oxygen precipitates
You have a rather perfect crystal with supersaturated oxygen, so basic homogeneous nucleation theory should apply. Well, yes, except that carbon, always
present in concentrations around 1 ppm, might induce heterogenous nucleation to some extent.
Whatever, if you want
serious nucleation you should use relatively low temperatures because that makes the critical radii smaller. Since you still
need some oxygen movement and don't want to wait forever, you can't go to extremely low temperatures.
Several hours
around 750 oC (1382 oF) might be the thing to do. |
|
 |
4.
Growth of oxygen precipitates
The small oxygen precipitates produced during the nucleation process are too small
for efficient gettering. Growing them to useful sizes at the low nucleation temperature takes far too long, so you now induce
a growth phase by going to a high temperature.
A few hours around 1000 oC (1832 oF) might be the thing to do.
|
 |
Now you are done. You employ what is known as the "High-Low-High" process
to produce the precipitate structure you need. Below are a few examples of what you might get, taken from the well-known
paper of D. Huber and J. Reffle. Diethart Huber (together with
Werner Zulehner) worked for Wacker, one of the few big Si crystal
makers in R&D. They are not only well known to me but to about everybody else in semiconductor science in Europe (and
beyond) because, besides being well-known silicon scientists, they supplied us poor university guys with all kinds of silicon
samples "just so". Thank you, Diethart and Werner! |
| | |
| |
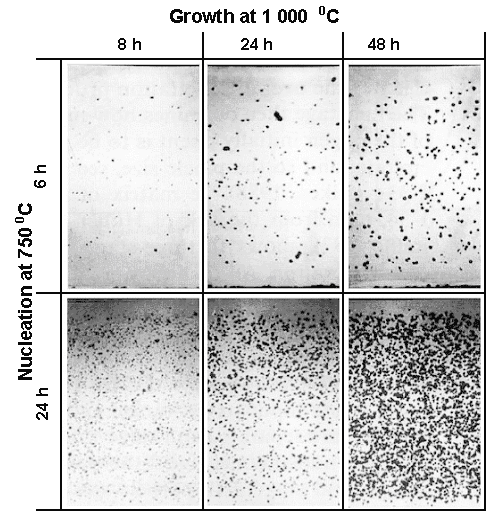 |
Development of SiO2 precipitates
Cross-section through complete wafer | |
Source: D. Huber and J. Reffle, Solid State Techn. 137 (1983) p. 26; by kind permission |
|
| |
| |
|
 |
The initial oxygen concentration was 8.15 · 1017 cm–3.
The precipitates were rendered visible by defect etching. Shown
are just a few out of many pictures for various parameters. Note the denuded zone obtained by a first outdiffusion treatment
at 1100 oC (2012 oF) for 10 hours.
The picture makes clear that details matter a lot. The
process you use for your product might thus be quite different from what I show here - and it's your secret! |
| |
| |
| |
The Parallels to Wootz Blade Making |
 |
The parallels to the making of a wootz sword are obvious, I hope. |
|
 |
What you start with is extremely important. Not all wootz steel cakes are the
same. You need some with a striated distribution of carbides
or carbide formers; just having 'hypereutectoid steel will not be good enough.
Nobody knows how that would turn into
a detailed specification list but we can be rather sure that it would contain a number of specific entries. |
|
 |
You need to forge it in such a way that the striated distribution of carbides or carbide formers
(like vanadium (V) or titianium (Ti)) is conserved in your blade. |
|
 |
The heat treatments you need for forging should promote the nucleation of carbides in the
striated pattern and their growth to rather large sizes. At the same time you must prevent at all costs the formation of
cementite shells around the grains of pearlite, the natural process that usually takes place. |
|
 |
If the temperature cycles you use for forging are not sufficient for achieving these goals,
you must treat the finished blade in a way that promotes Ostwald ripening.
You might use temperature cycling for this, carefully selecting the right temperatures and temper times or doing this (and
measuring the temperature and time by guessing). |
 |
Now you will have produced a blade that will exhibit the typical wootz pattern
after polishing and etching. |
|
 |
If you want to go beyond this and produce a wootz blade that
- shows a structure like "Muhammed's latter" in the pattern
- is particular hard, flexible, and doesn't break if bend to a semicircle,
you use secrets that we only can guess at. |
| | |
|
© H. Föll (Iron, Steel and Swords script)


