 |
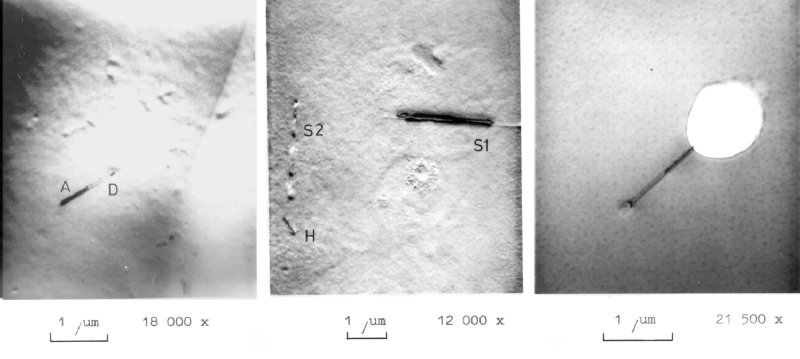
| Iron is a major contaminant in integrated circuits because there is a lot of steel in contact with the wafers or with materials needed to process a wafer. | |||
| Iron atoms diffuse as interstitials; they are rather mobile. Since the solubility at low temperatures is low, there is a strong tendency for agglomeration. The small iron silicide precipitates in turn serve as nucleation centers for large defects, especially the huge oxidation induced stacking faults. | |||
| An iron concentration of well below 1 ppb thus may enough to kill all integrated circuits in the thus "contaminated" part of a wafer. | |||
| |||
| The defects shown are almost certainly FeSi2 precipitates, which often occur in "needle-shape". Some stacking fault and dislocation dipole components may also be involved. These needles are already very large; the defects labelled "H" may be a smaller needle. | |||
![]() 6.3.2 Examples and Case Studies for Dislocations
6.3.2 Examples and Case Studies for Dislocations
![]() 6.3.3 Stacking Faults and Other Defects
6.3.3 Stacking Faults and Other Defects
![]() Oxidation Induced Stacking Faults in Silicon
Oxidation Induced Stacking Faults in Silicon
© H. Föll (Defects - Script)