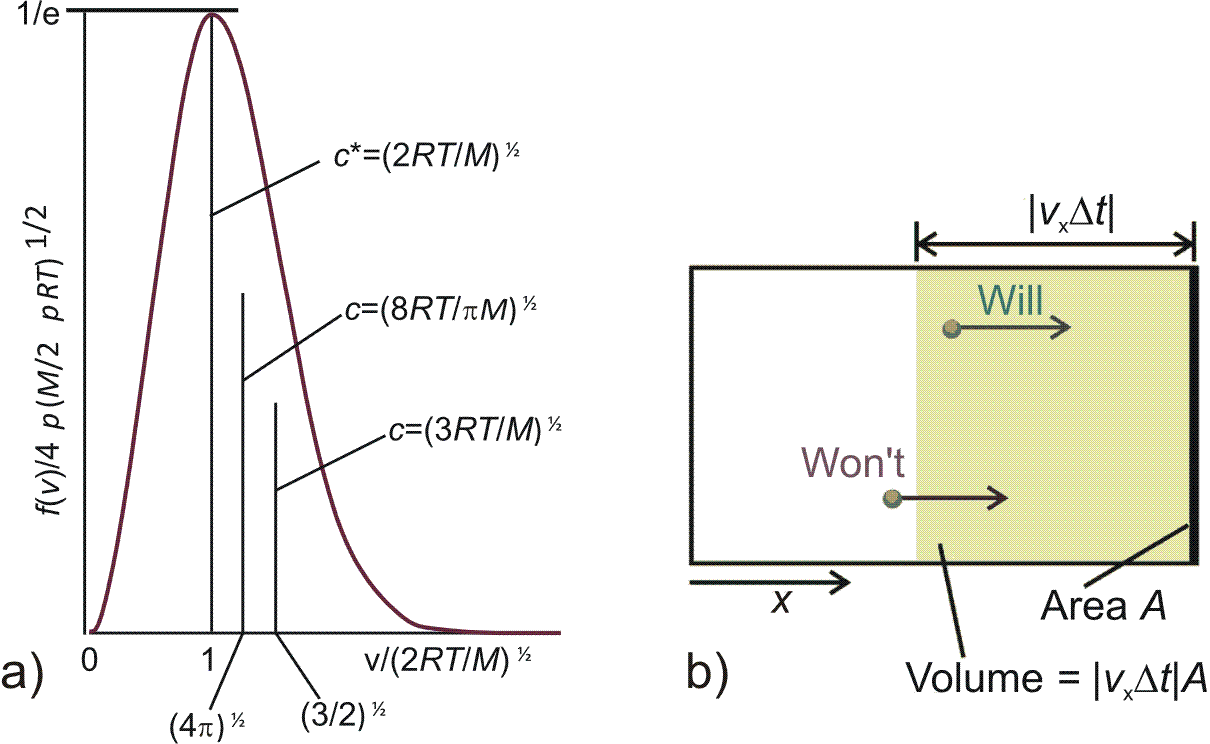
Special values for speed can be calculated for the Maxwell distribution
Mean speed
Mean square speed
Most probable speed
|
| \begin{equation*} \frac{df(v)}{dv} = 0 \quad \Rightarrow v_{max} = c^* = \sqrt{ \frac{2 R T}{M}} \quad . \end{equation*} | (4.22) |
Since within the ensemble all particles move one can define the relative mean speed of two distinct particles (which depends mainly on the masses of the particles)
|
| \begin{equation*} \overline{c}_{rel} = \sqrt{\frac{8 R T}{\pi \mu}} \quad \mbox{, with} \quad \mu = \frac{M_A \, M_B}{M_A + M_B} \quad , \label{Eq_relspeed} \end{equation*} | (4.23) |
which for the special case of identical particles (same masses) translates into
|
| \begin{equation*} \overline{c}_{rel} = \sqrt{\frac{8 * 2 R T}{\pi M}} = 4 \sqrt{\frac{R T}{\pi M}} \quad . \end{equation*} | (4.24) |

These special speed values are shown in Fig. 4.2 a).
In addition Fig. 4.2 b) illustrates the number of particles \(N_P\) with speed \([v_x, v_x +
dv_x]\), \([v_y, v_y + dv_y]\), \([v_z, v_z + dv_z]\) that will collide with the container wall
within the period \(\Delta t\)
|
| \begin{equation*} N_P = n^* A v_x \Delta t f(v_x, v_y, v_z) dv_x dv_y dv_z \quad . \end{equation*} | (4.25) |
So the pressure applied to the container wall is
|
| \begin{equation*} \overline{E_{kin}} = \frac{1}{2} m \overline{v^2} = \frac{3}{2} k T \quad , \end{equation*} | (4.27) |
the final result of Eq. (4.26) can be rewritten as
|
| \begin{equation*} p = \frac{1}{3} n^* m \overline{v^2} = \frac{2}{3} \frac{N}{V} \overline{E_{kin}} = \frac{N k T}{V} \quad , \label{p_2} \end{equation*} | (4.28) |
which is just the ideal gas equation.
© J. Carstensen (TD Kin II)