For the condition described by the Gibbs potential the partial molar volume \(dV_{p,T}\) is defined by the total derivative
|
| \begin{equation*} V_{p,T}(\alpha n_1, \alpha n_2, \cdots) = \alpha V_{p,T}(n_1, n_2, \cdots) \label{eq:V_hom_1} \end{equation*} | (5.11) |
(so, if e.g. doubling all particle numbers the system will obviously occupy the double volume, i.e. \(V_{p,T}\) is homogeneous in first order). We can now calculate the derivative with respect to \(\alpha\) in Eq. (5.11); applying the chain rule we get
|
| \begin{equation*} \sum_i \left(\frac{\partial V_{p,T}}{\partial \alpha n_i} \right)_{n_{j \neq i}} \frac{d(\alpha n_i)}{d \alpha} = V_{p,T}(n_1, n_2, \cdots) \label{eq:V_hom_2} \end{equation*} | (5.12) |
Since Eq. (5.12) holds for all \(\alpha\) we find for \(\alpha = 1\)
So the \(V(mixture)\) = sum of the partial molar volumes \(\times\) no particles for each component. The right hand version of this equation holds for molar volumes.
The volume of an ideal or heterogeneous mixture is \(V = \sum_i n_i V_{m,i} \)
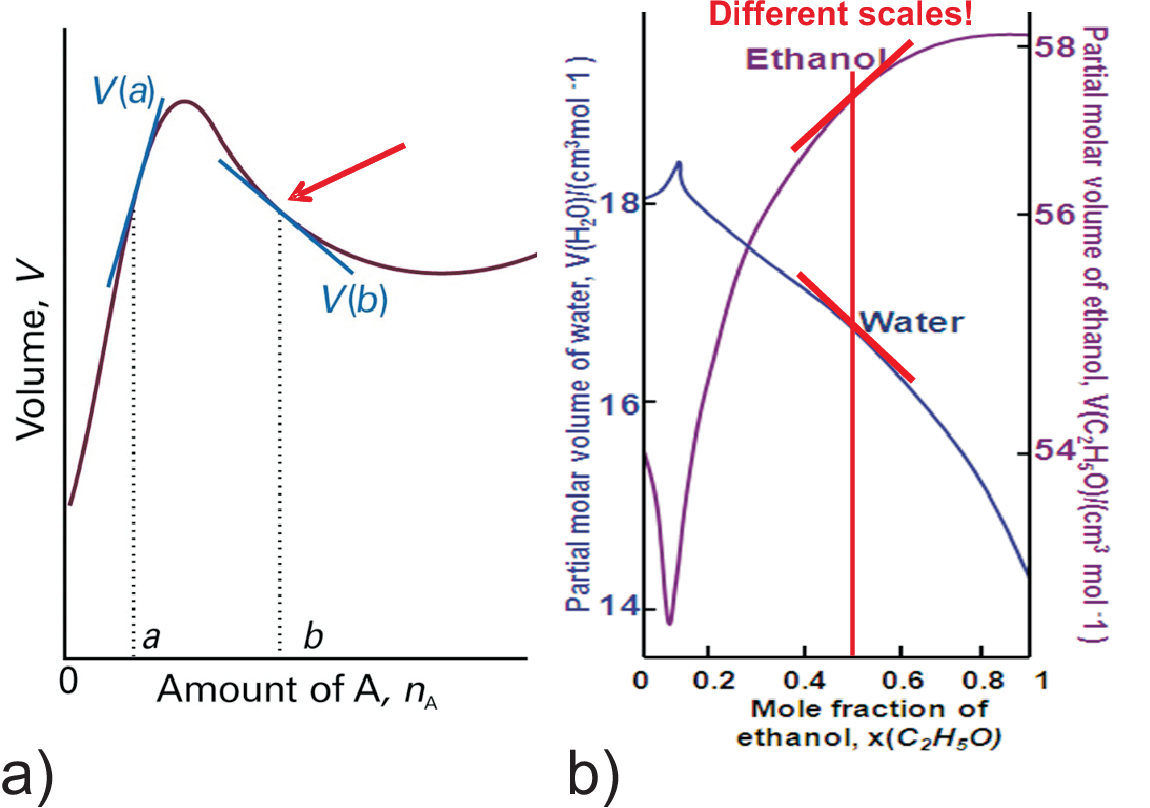
The volume of a real mixture depends on intermolecular forces, e.g. 1 ml ethanol + 1 ml water \(\neq\) 2 ml vodka! The mixing of ethanol and water gives about 1.92 ml. On a molecular scale this is interpreted by attractive forces dominant in the mixture, in terms of a more dense packing of the ethanol molecules (better H-bonding by water).
As a workaround the partial molar volumes are used which can be summed up (cf. ideal case) to give the volume of the mixture.
As an example we calculate the molar volume of a 50 at% EtOH [cm\(^3\)/mol]
mixture of water and ethanol. According to Fig. 5.3 b) the partial molar volumes of the pure substances are 18 for water and 58 for ethanol. Assuming an ideal mixture
we get
\(V(molar, mixture)\) = (0.5 \(\times\) 18) + (0.5 \(\times\)
58) = 38
Using the values for 50% we get the real value
\(V(molar,mixture)\)
= (0.5 \(\times\) 16.8) + (0.5 \(\times\) 57.5) = 37.2
This volume reduction
is a general finding: Except for very high water concentrations (left side) both molar partial volumes are always smaller
compared to the molar volume of the pure states (18 and 58). Thus the volume of the mixture is always smaller than that
of the ideal mixture.
© J. Carstensen (TD Kin I)