|
Light Emitting Diodes |
| | |
 |
Light Emitting Diodes or LED's are the cornerstone of optoelectronic products.
Very roughly they are used for two main product lines and for one speciality: |
 |
1. Signal lights All those little red, yellow,
green or blue lights (sometimes annoyingly), mostly used for
indicating that something has been turned on, is in a certain mode, or simply is just there (e.g. blinking red bicycle lights). |
|
 |
The main requirement for signal light LED's is that they come in many
colors (so designers of dashboards etc. are not limited
in their creativity; an important condition considering that designers appear to be limited in many other respects) and
that they are cheap. |
|
 |
Nowadays (2008) we do have all colors - including "white" -
but that was not always so. For generating a specific "color" including white you can go three routes:
- Take a semiconductor with the appropriate bandgap. This generates a "true" color, i.e. light around one wavelength
as given by our "master diagram" from before.
- Take semiconductors that generates UV or at least high-energy light and use it to excite some fluorescent material
- exactly like in fluorescent light tubes. That can produce white light or any color you find a fluorescent material for.
- Take three semiconductors that produce "RBG", i.e. red - green - blue,
in such an intensity mixture as to produce the color wanted - exactly at it is done by any screen or display.
|
|
 |
Obviously the first way is potentially the cheapest as long as you don't require
white light. That's why you mostly find LED's belonging to the GaAlAs family (red), GaP family (green)
or the nitride family GaAlN (blue to UV). Going from red to blue / UV also mirrors the history of LED
development. |
| |
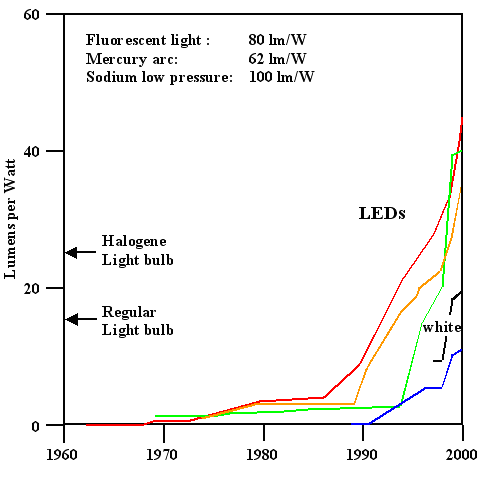 |
Source: M.R. Krames et. al., Applied Physics Letters, 75[16], pp. 2365, (1999)
and Scientific American, Feb. 2001 |
|
|
 |
The orange LED dscribed in the article mentioned above already had an
efficiency of > 100 lm/W, and today (Feb. 2008) 150 lm/w white LED's can be purchased, for example
from Nichia (the company that pioneered the blue / white LED). But now we moved already
into the second topic: |
 |
2. Light With the advent of the blue / UV
GaN-based LED in 1993 (that comes with a quite interesting story around its inventor, Shuji Nakamura and the company he was then working for (Nichia)), making white light with LED's
was possible for the first time. |
|
 |
For general lighting purposes - your room, the street, a congress hall, the street
in front of you bicycle or car, - you name it - you need first of all white light. After
you can do that with LED's, you need:
- High efficiency as measured by lumen / Watt (lm/W) or by
total "plug efficiency" in %, meaning the ratio of light energy out to electrical energy UIt
in.
- High intensity. It is not good enough to have a high-efficiency light source if
the best you can offer produces the same intensity as, let's say, a 20 W conventional light bulb.
- Large life time. You do not want to change your light fixtures too often. For
something in the better quality region, you want several years of operation time at the least.
- Low price. The price you can get for your white light LED depends on what
you offer. If it is much better than a regular light bulb, it does not have to be "cheap" - but it still must be worth its price.
|
|
 |
The picture below shows the efficiency of white LED's vs. existing light
sources: |
| |
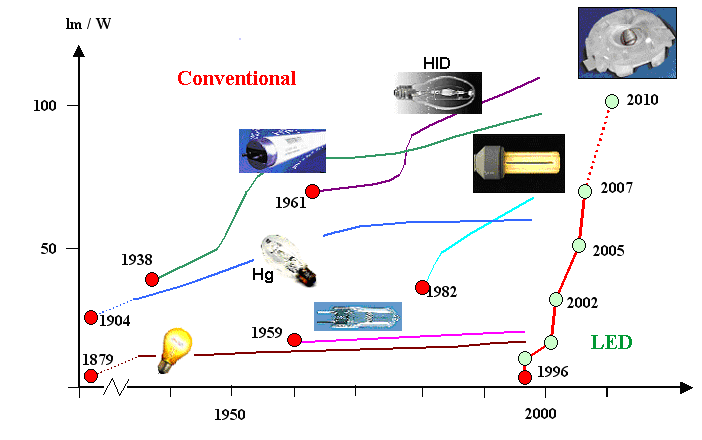
Based on information provided by Osram |
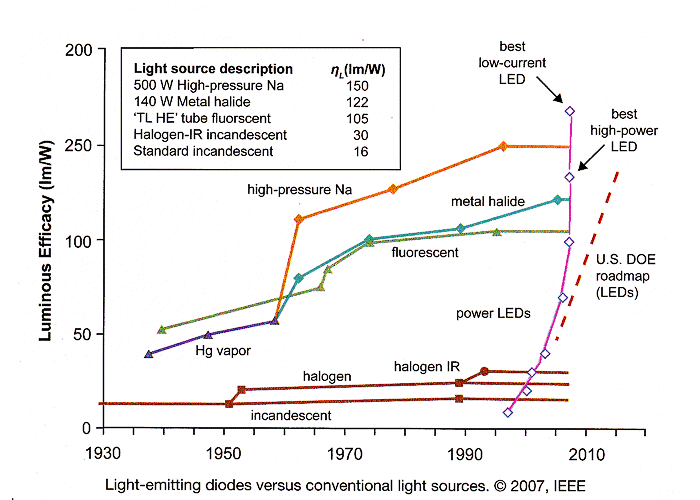 |
|
|
 |
The insets symbolize the type of "light bulb" sufficiently; the one
belonging to the purple line (the top performer of the "classical" light sources) is the metal halide "bulb",
belonging the the "high intensity discharge" (HID) type of light source. If you compare the development of the
white LED to all the other light sources, you get a first impression why everybody in the lighting business is so
excited about LED's as the light source of the future. Potential energy savings are enormous! |
 |
A quick word to the unit "lumen"
and to absolute efficiencies: |
|
 |
The "lumen" (lm) is the SI unit
of the perceived power of light; it measures the luminous flux.
Now the natural way to measure the flux of light would be to measure the energy flux
of the light. Since the eye, however, is not equally sensitive to the various wave-lengths it can see, the lumen corrects
for this. |
|
 |
As far as converting lm/W into absolute efficiency goes, we have the following
approximate relations - (10 - 15) lm/ W Û (5 - 9) %
- (70 - 100) lm/ W Û (25 - 35) %
|
 |
With respect to efficiencies, LED's do have a bright future, indeed. What
about the other criteria? |
|
 |
Product life time is not a problem but an asset. Well-made LED's will
last for >10 years, outperforming more or less all other light sources. |
|
 |
The problem is: intensity. The light is typically
produced in a small volume (great for focussing etc.), and if you put a power of let's say 100 W into a volume of
<1 mm3, you better have some concept of keeping the temperature down. |
|
 |
Closely related is the problem of plug compatibility, meaning that you want to
use 230 V 50 Hz AC, 110 V 60 Hz AC, or whatever your country has as its consumer electrical energy standard.
A LED, however, is a forwardly biased pn-junction, running at something like 3 V DC (and then 33
A if you want 100 W). While this is an electrical engineering problem, it is still a big problem. |
 |
The
(inorganic) materials used for both applications (always as thin layers) are once
again: |
| |
- Aluminium gallium arsenide (AlGaAs) — red and infrared
- Aluminium gallium indium phosphide (AlGaInP) — high-brightness orange-red, orange, yellow, and green
- Gallium phosphide (GaP) and Aluminium gallium phosphide (AlGaP) — green
- Gallium arsenide phosphide (GaAsP) — red, orange-red, orange, and yellow
- Gallium nitride (GaN) and Indium gallium nitride (InGaN) — near ultraviolet, bluish-green and blue
|
| | |
|
|
Laser Diodes |
| | |
 |
Semiconductor
Lasers will be treated in some more detail in module 9.2.2. Here we simply note
that the theory of (semiconductor) Lasers is rather complex, but the technology is not. |
| | |
|
 |
In principle, many LED's "automatically" become a Laser if you run a very
high current through them (producing a lot of light) without destroying them first. It is therefore not too difficult to
produce a Laser diode in the Lab - all you need (haha) is very efficient cooling of your experimental LED device. |
|
 |
| Laser and optics of CD player. |
|
|
 |
You may know already that Lasers always need some kind of optical feed back, usually provided
for by mirrors, and ask yourself where the mirrors are if we use a simple LED as Laser. The answer is that plan-parallel
surfaces of the semiconductor might be already sufficient for that because the interface semiconductor - air does act as
a "semi"-transparent mirror and that might be good enough. |
|
 |
The truth, however, is that it often takes many years after a certain new LED
has been marketed, before the long-lived, reliable and cheap Laser diode follows. |
|
|
 |
The first GaN-based blue LED's were on the market around 1993, whereas
the blue GaN based Laser had to await 2005 or so (in 2006 SONY, for example, still had major production
problems). | |
 |
Using semiconductors for making a Laser is just one way for making Lasers - you
can use other solids, liquids and gases for that. This brings up the question of pro and cons - what are the advantages
and disadvantages of semiconductor Lasers? | |
|
 |
Look at CD and DVD or now blue ray disc drives
- the are the major market for semiconductor Lasers besides the very pedestrian "Laser pointer". The advantages are obvious:
| |
|
| - Very cheap.
- Very small.
- Electric energy supply at low voltage.
| |
|
 |
The major disadvantages are | |
| |
- Low power at decent quality (around 1 W maximum).
- Limitations as to color (= frequency).
| |
 |
The cheap and reliable semiconductor Lasers are actually the enabling devices for all this memories! No suitable Laser - no discs. |
|
|
| |
| |
 |
Sorry - your Laser pointer just can't be turned
into the Laser gun you might fancy for fighting those aliens if you are a male (or,
in your adult life, for cutting metal or other materials). For this you need other Laser types which can deliver real power - far heavier, bulkier and far more expensive than your semiconductor Laser. |
|
 |
Of course there are constant optimizations and new developments -
newer and better semiconductor Lasers are frequently announced. We haven't seen the last of this yet. |
| |
| |
|
|
Displays and OLED's |
| | |
 |
Take a million or so LED's, arrange them in a matrix, and make sure they
can be individually addressed - you now have a display. |
| |
| |
|
 |
If you make your display with individual LED's, soldered together somehow,
you will get an expensive big display with lousy resolution - the kind of boards you see on Times Square or other places
that have been taken over by the evil advertising people. | |
|
|
 |
If you could make your LED's tiny
in the lateral direction and all of them close together, i.e. on one
substrate, and for all three RGB colors and
individually addressable, you would have a flat panel display that would be
great for TV, computers, cell phones and laptops because it could have a high energy efficiency. |
|
 |
Unfortunately, the possible substrates for inorganic semiconductor LED's
are far too small (we have only the III-V single crystals, essentially GaAs, SiC and perhaps Al2O3
(= Sapphire)) and those potentiial substrates do not come even close to what would be required. |
|
 |
Fortunately, an unexpected discovery made accidentially in the 70ties by
Shirakawa in Japan (and to some extent by others before him)
has helped: | |
|
 |
There is such a thing as an organic conductor,
and an organic semiconductor leading to an
organic light emitting diode - an OLED. | |
|
 |
OLED's have only be around for less then 5 years, but
we already have flat panel displays based on OLED's in cell phones and the first ones for TV are announced
right now. |
 |
Let's be clear about one thing: Organic semiconductors right now are still lousy semiconductors (and extremely sensitive to oxygen).
|
|
 |
They have a tremendous advantage over inorganic semiconductors, however:
they can be very cheap and, far more important, they can be deposited at low temperatures by rather simple techniques on
cheap and very large substrates and they are easy to pattern. |
|
 |
In other words: OLED displays are easy to make, the light
emission is OK, and the product life time is OK at present for consumer items where demands are a bit more
relaxed. |
| |
|
 |
What we are witnessing right now is the beginning of a completely new field of
semiconductor materials science and technology. Who knows where it will end! |
© H. Föll (Semiconductor Technology - Script)