|
|
For a large number of molecules and solids the ground state is a singlet state of Eq. (4.68) and the descriptions of the wave functions
following the Eq. (4.77)-(4.79) are a good approximation. Both electrons occupy the
ground state \(\psi = \frac{1}{\sqrt{2}} \left(\Phi_1 + \Phi_2 \right)\) with opposite spin.

|
|
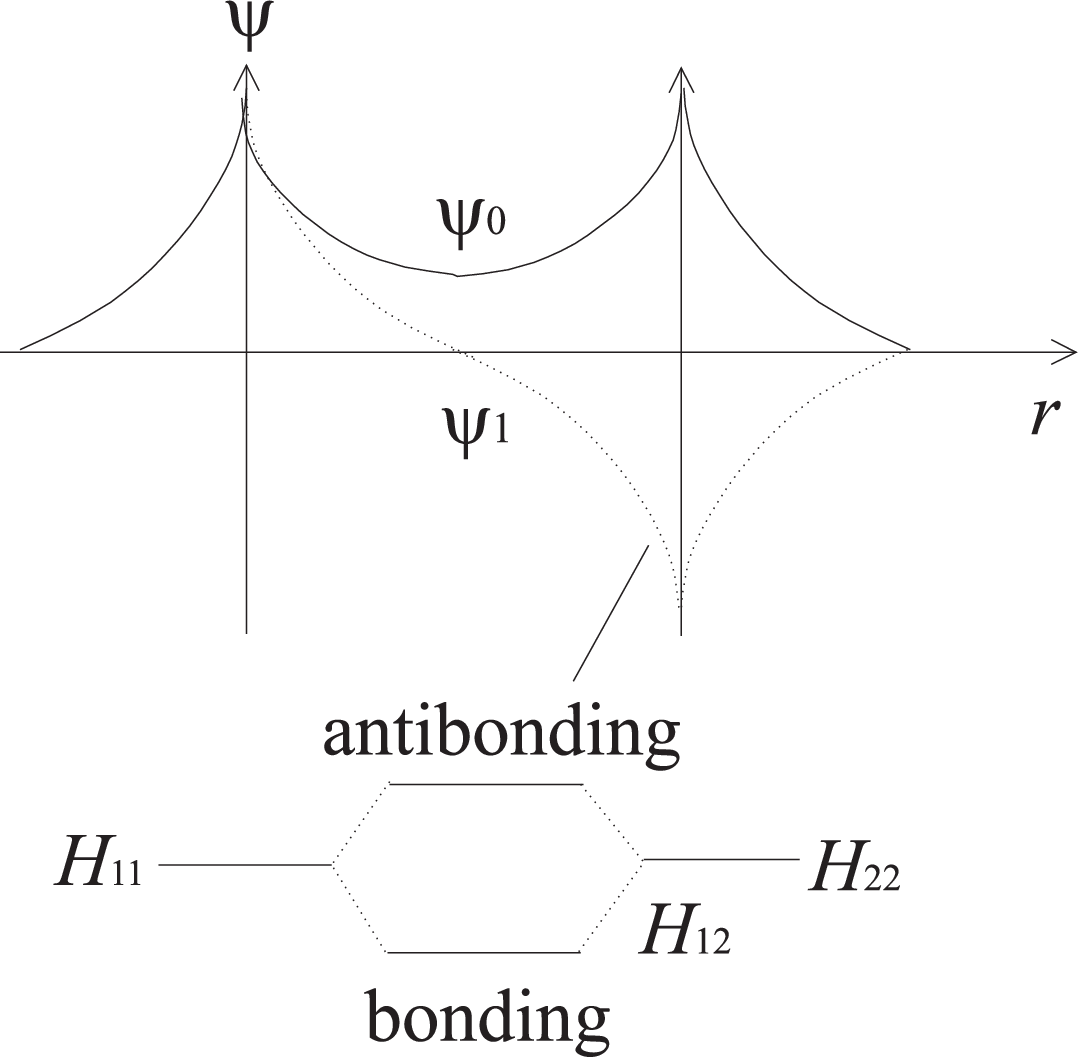
Two electrons from two atoms of equal electronegativity share one state which is composed from atomic orbitals.
Only atomic orbitals which are not fully occupied can use covalent bonding, since the binding orbital can only take two electrons.
The corresponding binding energy is according to Eq. (4.76) defined by the overlap integral \(H_{12}\).
Covalent bonding are directed, since this allows for the optimal overlap.
To maximize the overlap and the number of bonds often the atoms form hybrid orbitals. The necessary excitation energy is overcompensated by the increase of the bonding energy. The hybridization allows for a great variety of mixed states which in consequence allows to optimize the overlap of atomic orbitals.
The symmetry and structure of the crystals is dominated by this bonding with a strong direction.
The essence of the covalent bonding is the reduction of the coulomb repulsion of both atoms by increasing the electron density between the cores. But this allows a reduction of the energy only if the coulomb repulsion between the electrons does not overcompensate this effect.
© J. Carstensen (Quantum Mech.)