| |
Free Enthalpy of Reduction or Oxidation Processes |
 |
This module augments the advanced module "Free
Enthalpy of Reduction Processes". It shows in particular the free enthalpy gain vs. temperature diagram in more
detail. Most everything of interest is explained in the advanced module. Smelting, i.e. the reduction of the oxide, only
occurs if the reaction line is above the carbon monoxide line since it is the difference in energies that counts. |
|
 |
Here I only want to point out a few things:
- Iron oxide reduction runs in several steps:
Fe2O3
—> Fe3O4
Fe3O4 —> FeO
FeO —> Fe
Together
it takes far more energy to generate iron (Fe) than, for example, copper (Cu).
- The final step, the reduction of FeO, only gains energy by transferring the oxygen to the carbon monoxide above about
750 oC. FeO melts before Fe does.
- Smelting lead (Pb) or zinc (Zn) takes more energy than smelting copper, despite their low melting points.
- Zn produced above the smelting temperature will become completely vaporized.
- Smelting nickel (Ni) and cobalt (Co) should be easier than smelting iron (Fe).
|
|
 |
You just as well can read the diagram "backwards" and find out, how much energy
will be released if you bubble oxygen through molten iron, i.e. at temperatures above the red vertical line.
|
| | |
|
|
|
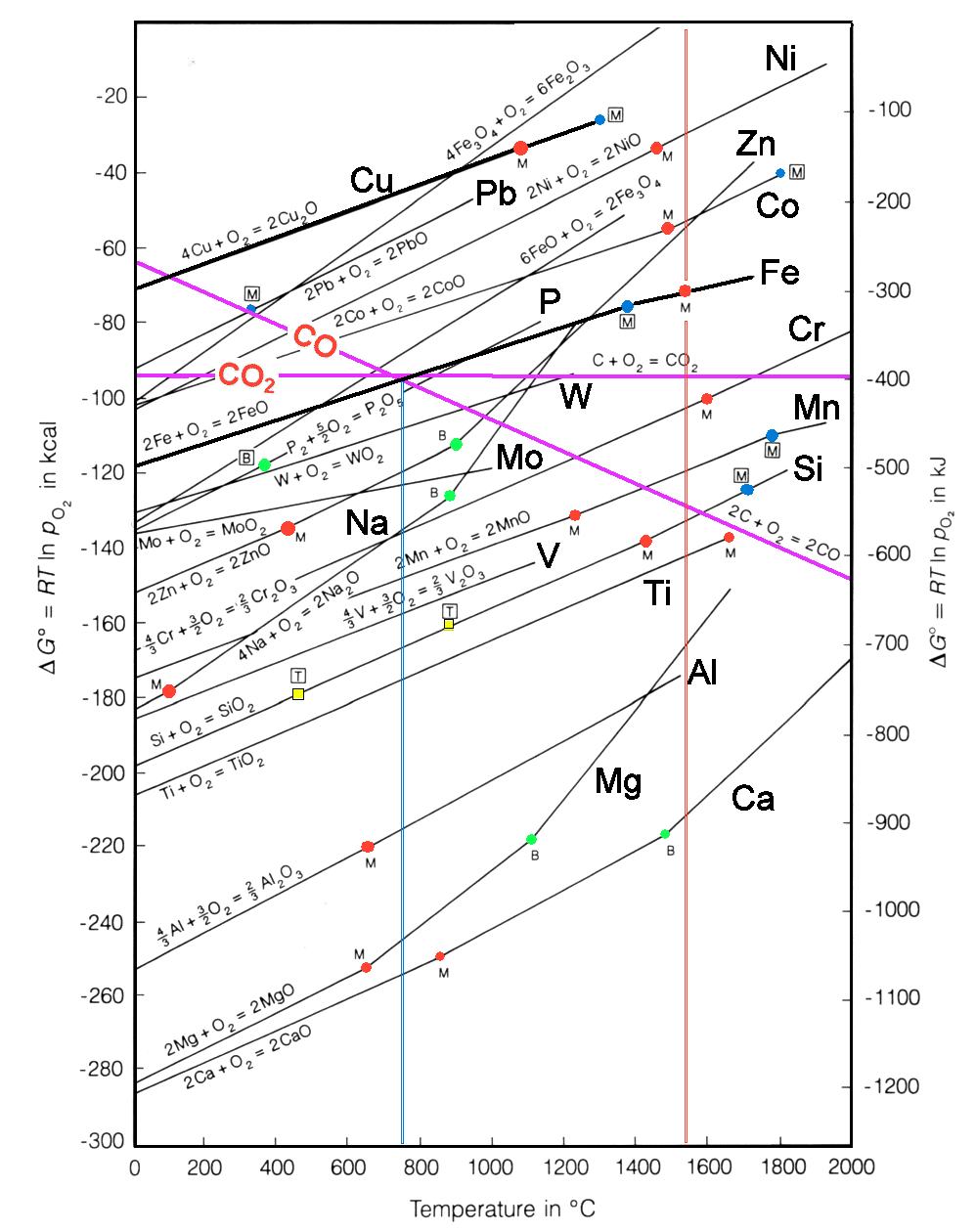 |
Energy gains / losses in forming / dissolving oxides as a function of temperature
(Click to enlarge)
|
 Melting point of metal; Melting point of metal;  Melting point of oxide; Melting point of oxide;  Boiling point of metal; Boiling point of metal;  Phase transition point Phase transition point |
|
| | |
|
© H. Föll (Iron, Steel and Swords script)
