|
10.5.3 Making Steel after 1870 |
| |
A Few General Words |
 |
The year 1870 is as good as any in the second half of the 19th century to mark
the turning point in steel production or simply a major revolution. And if
I use the word "steel revolution" I include wrought iron and cast iron because
if you revolutionize steel production you must start with cast iron and wrought iron.
The revolutionizers of steel
in the second half of the 19th century were more concerned about the quantity of iron
and steel produced and not so much about the quality of the product. The industrial revolution taking place right then called
for unprecedented quantities of iron and steel. Just consider how much of the stuff you needed in
addition to everything else when you set out to establish a railway system! "Railway", by the way,
translates to the German "Eisenbahn" (=iron path) or the French "chemin de fer" (=iron road) for good
reasons.
There was no problem with increasing the production of pig iron. Blast furnaces could be made bigger and bigger
and easily produced tens or even hundred of tons
of pig iron per furnace and day. The costs actually decreased because you had substantial economy of scale effects. The big problem came with the fining
needed. The available processes for making wrought iron by "fining" or steel by puddling, cementation, crucible,
whatever, produced a few hundred kilograms or at most 0.7 tons per unit and day. If you wanted to increase production you
needed more furnaces; you couldn't make them bigger. There was thus no economy of size and that means no cost digression.
Right now we have a similar situation with respect to refining raw silicon needed in exponentially increasing quantities
for solar cells.
Bessemer consequently stressed that he
could manufacture malleable iron and steel without fuel, i.e. he emphasized a cost issue
and not that he could make better steel. But as ever so often quantity and quality is interrelated, and the new methods
impacted the quality of the products just as much as the quantity / cost issue. You certainly cannot build a railroad if
the material costs of the rails are prohibitively large. But you also cannot operate a railroad for very long if there are
too many gruesome accidents because rails fracture too often
due to the fluctuating quality of the iron / steel. And you don't even need to consider erecting highrisers if there is
the slightest doubt about the quality of the elevator cables.
The final result of the steel revolution was that the
quantity of quality-guarantied steel produced in new ways started to grow exponentially
ever since. What happened is that the steel revolution allowed new products that in turn caused major changes of their own.
Reliable, safe and cheap railroads allowed to transport people and products far more quickly and cheaply than before, which
allowed to open up vast stretches of so far undeveloped areas like the "West" of the USA, mixed people of all
kinds of backgrounds, bred social problems and improvements, and so on and so forth. The car industry is unthinkable without
steel. In short, an avalanche was started that is still gaining momentum and power - just look at the development of the
World's steel production below. |
| |
 |
| Worldwide Iron / Steel production since 1870 |
| Source: Blue curve:
"Werkstoffkunde Stahl"; Red curve: Internet a large |
|
|
 |
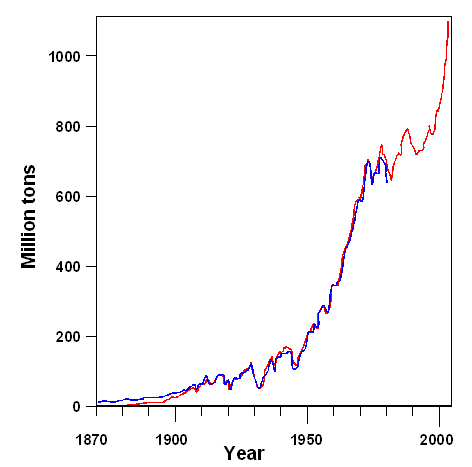
In march 2018 the President of the USA, one Donald Trump, imposed import taxes
on steel. One of the reason is that China now dominates world-wide steel production. According to newspaper, the production
capacity is 2.4 billion tons (or be 2400 million tons in the figure above), with China accounting for about one half. However,
only 1.5 billion tons are needed, resulting, as always, in some kind of trade war if not a real one. |
 |
One cannot possibly overestimate the importance of steel for the development of
humanity in the second half of the 19th century and most of the 20th century. Just a very few reminders. Without affordable
steel, having specific and reliable properties, you would not have:
- Buildings with more than 5 floors. Elevators need steel cables.
- Railways, large bridges, cars, big ships or simply affordable transportation.
- No airplanes either. While they are mostly not made from steel, they couldn't be made without steel tools. They also
need some crucial steel parts e.g. in the engines and the landing gear.
- Same thing for electronics. Chips are made from silicon, alright, but chips are
made with plenty of steel tools.
- Without steel no tractors, harvesters, plants for making fertilizers, and thus no agricultural revolution. No agricultural
revolution and most of us would be dead or would never have been born because of world-wide famines.
- No big cannons, machine guns, nuclear reactors, atom bombs without steel.
- And so on.
However, mastering steel is not sufficient for starting an second industrial revolutipon. It is just the first major ingredient definitely needed. The second absolutely
necessary ingredient for the industrial revolution was cheap energy in the form of coal and later oil plus energy transferring
devices like steam engines or gasoline motors. The third - and that is a surprise for
most - is rubber. Think about that yourself and you will see why.
If you have all that, you can finally go into electricity
and make the really big changes. |
 |
Of course, a lot of other things happened around 1870 and later that also changed
the world in major ways for better or worse. The Germans invaded Gaul (then called France), for example. Foremost in my mind, however, is the scientific revolution
giving us, for example, electricity. However, no electricity without steel. Things are interconnected. This is as trite
a statement as one can make, so let's stop here and just give a very brief look at some of the key breakthroughs made in
the steel revolution. I will do that by looking at some of the guys whose names are attached to these developments. Biographical
details can be found here. |
|
 |
This is doing grave injustice to many other scientists and engineers. All big
names in science and engineering owe their success to a smaller or larger extent to their colleagues, including the already
dead ones. There are no true scientific giants. Some scientists and engineers are (intellectually) considerably taller than
the average but they are not giants. They stick out of the crowd because the stand on the shoulders of all the others that
form a kind of intellectual pyramid.
More details about the "steel revolution" and other key people involved can be found in the link.
| |
|
| |
| |
| |
The Bessemer Process |
 |
On August 13th, 1856, Henry Bessemer presented a paper entitled: "The manufacture of malleable iron and steel
without fuel" to the British Associations for the Advancement of Science, an important organization. The paper described
that by blowing air through molten pig iron, the carbon, silicon
and manganese in there were removed, making the iron malleable.
Before that, between Oct. 1855 and Feb. 1856, Bessemer
had filed for 3 patents relating to running air or steam over or through molten pig iron. What exactly was going on on his
mind, what he found out by his own experiments, and to what extent the was aware of the work of others, is a long and muddled
tale; see the link for some details. The obsession of Bessemer and all
the others with filing plenty of patents just makes clear once more: the steel revolutionaries were primarily concerned
about making money and not all that much about advancing science and engineering. |
|
 |
Bessemer must have known the basics about the role
of carbon in iron and that it could be removed by oxidation even so he coyly pleaded ignorance of iron metallurgy. Note
that his paper emphasizes that he can do things "without fuel". Note also
that the idea of blowing air through a large quantity of molten cast iron may not be seen as all that innovative. It carries
a few connotations, however:
- You need real power for blowing and something better than bellows for producing the air stream.
- You need novel high-temperature prove hardware because the temperature went up quite a bit from the melting point of
pig iron.
- You need guts because the idea is preposterous. Blowing cold air through liquid
cast iron will simply solidify it, of course. At least that's what one would expect.
The third point is what most people thought. Bessemer claimed that the melt actually get's hotter!
Not quite believable but Bessemer was right. The energy released by all the oxidation reactions was sufficient to keep the
stuff liquid even so the melting point goes up to a whopping 1538 oC (2629 oF), making point 2 hard
to meet. "All the oxidation reactions" include in particular the oxidation of silicon and phosphorous contained
in many but not all pig irons because these reactions deliver a lot of energy, and the oxidation of iron itself. Here we have a first hint that the success of Bessemer's process may depend critically on the precise
composition of the pig iron. |
 |
In the 1856 meeting Bessemer did manage to convince a number of iron masters present
that his invention was of interest. Licenses were taken and large-scale experiments were started right away. After all,
there was a real potential to "fine" tons of pig iron within 30 minutes or so, a tremendous increase in productivity.
Within just a month, the honeymoon was over. A big problem had emerged: |
| |
The Bessemer process produced
shitty iron & steel
|
|
|
 |
The iron produced by using Bessemer's methods was completely de-carburized wrought iron but
cold short as well as hot short, and "oxidized to a cinder"! There were a number of reasons for this severe disappointment,
details are here.
In short: The process had worked on a kind of
lab scale but not on a production scale. The major reason was that normal English ore
(in contrast to the "lab grade" ore Bessemer used) contained phosphorous and the Bessemer process could not get
rid of phosphorous.
This problem could be circumvented by using phosphorous-free
ore, making suitable pig iron. Unfortunately, English ore didn't qualify. That caused caustic comments by Bessemer's
contemporaries. For example J. Percy, a renowned authority on iron
and steel making, remarked in 1864: "....it is only pig iron practically free from phosphorous
that Bessemer can deal with satisfactorily and such pig iron forms merely a fraction of the total produced in this country.
It will be time enough for Mr. Bessemer to sneer at puddling when he can show how that laborious operation may be dispensed
with. He has not yet done so."
Swedish pig iron "worked", however, as was more or less accidentally
discovered during the frantic work Bessemer and others invested for about 18 month. It just tended to "blow cold"
(not staying liquid) because besides phosphorous it also lacked silicon and thus a booster of temperature.
The next
problem that came up was the production of steel. Taking out the carbon completely (i.e. making wrought iron) was no problem
anymore but there was no way to stop the process at precisley the the right moment to retain a defined concentration of
carbon as needed for steel. The solution was to re-carburize the melt with "spiegeleisen",
a manganese - iron - carbon alloy that some Germans made. Robert
Mushet, a big name in iron / steel, had patents on this and some
cooperation was needed. Spiegeleisen (literally "mirror iron"), via its manganese, also took care of the sulfur and thus of hot shortness
Finally, Bessemer could produce good
iron / steel also with "native" (Lancashire and Cumberland) pig iron that happened to be low enough in phosphorous
and sulfur and the first mass production could start. |
 |
Bessemer converters became a common sight and accounted for about 30 % of the
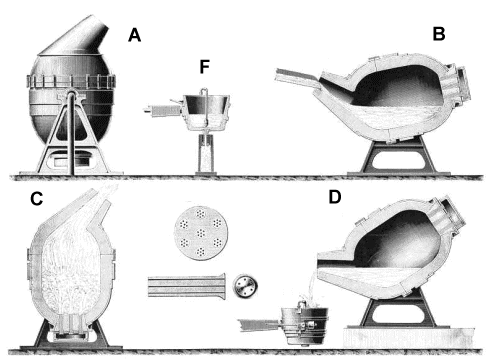
World's iron / steel production between 1880 and 1900. The converter itself was a marvel of engineering; here is a picture of a real one. The picture below shows drawings from an old illustration of uncertain origin.
|
| |
|
|
 |
What we see is: - A: A rendering of the converter.
- F: The ladle into which the finished iron is poured.
- B: The converter being filled with liquid pig iron.
- C: Blasting air through the liquid.
- D: Pouring the finished product into the ladle.
- Between C and D: Details of the bottom with the tuyeres.
It goes without saying that the lining of the Bessemer converter with some refractory material was crucial. The ceramic
refractory bricks must have been able to take the heat / high temperatures and the
extremely corrosive environment without having to be replaced too often. Injecting the air with sufficient pressure to make
it through the heavy liquid, and controlling this to some extent, wasn't an easy task either.
More details about the
Bessemer process can be found here; and here
is a big picture of the real thing. |
 |
The phosphorous problem was "solved" by using phosphorous-free pig iron.
That was not a real solution, of course. The real solution can be found below under the heading "Thomas
Steel", an important variant of the Bessemer process covered below. |
|
 |
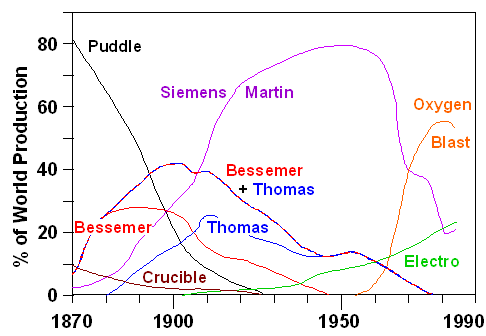
Around 1892 the total amount of iron and steel made with the Bessemer process
and the similar Thomas process surpassed the production by puddling.
The data below show this. They also show that another invention, the Siemens - Martin process,
overtook Bessemer in 1900 and everything else in 1910.
We also see that making steel with Huntsman's
crucible process or variants thereof declined synchronously with puddling. Note, however, that all the new processes
contain the essence of the crucible process: they produced liquid iron or steel that
could be cast. |
| | |
| |
|
|
 |
Before I deal with the Thomas process and the Siemens - Martin process, I will
give another quick look on the scale of the iron / steel production in the late 19th century. The "big" picture shown above gives the impression that not much iron and steel was produced
between 1870 and 1900. That is wrong! It's just a matter of scales! So let's look at what really was going on in those years: |
| |
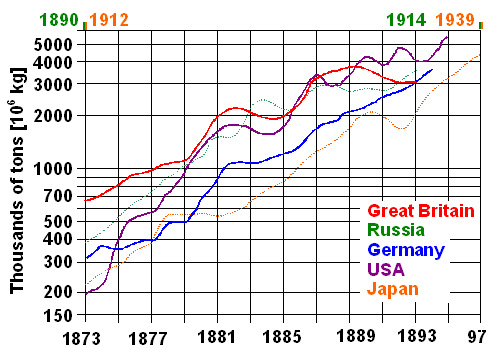 |
Early iron / steel production in some key countries
Note logarithmic scale and change of time scale for Russia
and Japan
|
| Source: Redrawn from the curves given in Wertime's
book. |
|
|
 |
All curves increase linearly on average, indicating exponential growth.
All curves have roughly the same slope, indicating a ten-fold production increase about every 20 years or a yearly average
growth rate far in excess of 10 %. You just can't install all the infrastructure needed more quickly, it seems.
1892,
when the Bessemer / Thomas processes overtook the puddling process worldwide, Great Britain alone produced about 3 million
tons of iron / steel. In 1870 it was about 500 000 tons, still quite a bit - and roughly 50 000 tons were already made with
the Bessemer process that came on-line just about 10 years earlier. It just takes some time (not to mention lots of money)
to build he heavy-duty infrastructure required with many novel components and considerable risks for the investors. |
| |
| |
| |
The Thomas
Process |
 |
Many iron ores contain some phosphorous and so does the pig iron smelted from
them. Blowing air through liquid pig iron obviously did not remove the phosphorous. It neither bubbled out as a component
of a gas like carbon, nor did it become part of slag or dross swimming on top. The reasons for this are actually quite involved;
here is a short discussion.
If you want to get rid of the phosphorous,
you must make it part of some slag. The Bessemer process did produce slag. The oxidation of iron produced FeO, known to
us by now as wüstite, and the oxidation of the silicon produced SiO2.
Together you get the ubiquitous fayalite - wüstite slag well known
from millennia of smelting. Phosphorous would be oxidized to P2O5 during the air blast but would not
be able to compete with the SiO2 in the reactions needed for forming the stable slag ingredients. It would be
reduced again by the CO formed in large quantities and then remained in the pig iron.
The only logical way to remove
it thus was to "catch" it with something else. Limestone (calcium carbonate, CaCO3) added as flux could
do the trick but it would also corrode the refractory silica-bearing bricks lining the converter.
With hindsight (and
some knowledge of high-temperature chemistry) it is relatively clear what happened but in 1870 things weren't that clear.
|
| |
For obscure reasons, the words "acidic" and "basic" come up
a lot in this context. What that means is bisected in some detail here;
suffice it to note that the normal bricks, always containing some silicate (something ending with SiOx) were
acidic and could not deal with basic limestone or quicklime.
|
|
 |
What kind of brick should one use for the lining? Without compromising the primary
job of the lining like mechanical and chemical stability in a very hot and corrosive environment. Not to mention that platinum
bricks (they migt work) or something else very expensive wouldn't do either. One Sidney Gilchrist
Thomas, a civil servant with an interest in iron making and metallurgy, took the bit in
his teeth and set out to solve the biggest problem of the 1870 iron and steel industry. He enlisted the help of his cousin
Percy Carlyle Gilchrist, a chemist who worked for an iron making
company. They worked hard but had no luck for several years. Eventually the iron mill Gilchrist worked for gave them some
support and they started experiments with a small lab-type converter, trying all kinds of materials they selected on "theoretical"
grounds. They finally succeeded, announcing the Gilchrist - Thomas process during a
meeting of the Iron and Steel Institute in London, March 1878. |
 |
They didn't hit it off a first. Almost nobody was ready to believe that two amateurs
had beaten scores of professionals who worked hard at solving the biggest mystery of the iron industry in the 1870ties:
how to process phosphorous containing iron. Finding a way was a big issue because it would allow to use the phosphorous-bearing
ores that formed the vast majority of the known ore deposits.
Believes and doubts are one thing, an actually working
process is another. The Gilchrist - Thomas process worked - and fame and wealth eventually descended on the two.
What
was the big trick? It looks actually deceptively simple. Just line your Bessemer converter with bricks made from dolomite, a calcium magnesium carbonate (something like CaMg(CO3)2). Dolomite
is a rather common rock; parts of the Alps consist of it. "Calcining" (heating) it produced calcium and magnesium
oxides. The stuff was ground up, mixed with dry tar, pressed into bricks and used for lining a converter. Now add a fitting
amount of burned lime ("quicklime") as flux to
your molten pig iron, and your phosphorous will end up as calciumphosphate (Ca3(PO4)2 in
the (easily removable) slag that forms now - while your lining will not be attacked. |
|
 |
It was actually necessary to have quite a bit of phosphorous
in the melt for the process to work. The Gilchrist - Thomas process wasn't quite that simple, after all. Once more, success
depended in doing a lot of things just right. |
 |
The Thomas process became far more important than the Bessemer process since 90
% or so of the world's pig iron is phosphorous rich. Don't be deceived by the percentage data above
where the Thomas process has about the same percentage as Bessemer. In 1960 a certain percentage meant a hell of a lot more
production in tons than the same percentage in 1920, for example.
The Thomas process allowed Germany, Sweden, France
and others to use their ore deposits for making large quantities of iron and steel. The production peaked around 1910, 20
years after the Bessemer heydays - and by then the world production had increased at least 10 fold! |
|
 |
As an unexpected fringe benefit it turned out that the phosphate-rich slag was an excellent
fertilizer. "Thomas
flour"
was the first synthetic fertilizer and found a big and receptive market. It was still used in the 1950ties and 60ties in
the farming town I grew up in. Here is a commercial from around 1940 |
| |
 | | Advertising for Thomas flour |
|
 |
However, while Bessemer and Thomas most certainly were great inventors and remarkable
personalities, who left an indelible mark on the iron and steel history, the world at large could have done without them.
The competing Siemens - Martin process came in only a little later and was so much better that it dominated the industry
for a long time as the percentage figure above nicely demonstrates. |
| |
|
| |
The Siemens - Martin
Process |
 |
The Siemens - Martin Process is also known as "open
hearth" or "regenerative furnace" process. Let's start
with Mr. Siemens' contribution. The first thing to know is that there was not just one Siemens involved but a tightly knit
big bunch of them. The two Siemens brothers
most important in this context are :
- Carl Wilhelm Siemens; later Sir
William Siemens (1823 – 1883, knighted 1883) left for London in 1840 as an agent of his brother Werner, the founder of what is now Siemens Corporation.
He stayed in England and became a knighted British subject. He is the one mostly associated with the Siemens - Martin process.
- Friedrich August Siemens (1826 - 1904)
joined his brother Wilhelm for a while in England. He went back to Germany in 1857 or so, employing the new technology with
great success in the glass industry.
|
|
 |
Sir William was scientifically minded and used his knowledge to come up with
the regenerative principle for furnaces. Or did he? During the crucial time around 1847 his brother Friedrich worked with
Wilhelm; and it is actually Friedrich who got a patent.
If Wilhelm's or Friedrich's contributions was more important I do not know. The Siemens' brothers however, never produced
steel themselves with their invention.
The Martin's (father an son) in France were the first ones who got a licence,
and their work was instrumental for finally coming up with a working Siemens - Martin
furnace (see below). |
 |
What is it all about? Well, at least Wilhelm's mind was full of the new insights
from a scientific revolution in the field of what we now call thermodynamics, tied to
names like Carnot, Clapeyron, Joule, Clausius, Mayer, and Thomson. In particular he accepted the new and revolutionary notion
that heat was not a substance but a form of energy. |
|
 |
As far as I can make out, he (or both brothers) used the new scientific knowledge to figure
out answers to two simple questions: - How can I produce very high temperatures ....
- ... with as little energy = fuel as possible?
One could now calculate how much you can achieve with a given amount of energy. In reverse, it also was possible to
calculate how much of the energy actually used for achieving something like the melting a certain amount of iron was actually
wasted, not really needed. It must have become clear to Siemens that a lot of the energy used in iron processing simply
went down the drain; it was wasted. What could be done about this?
The catch word was and is"regenerative energy". That means to employ energy usually wasted for doing something useful. You
may brake your car electrically for example, using the energy that otherwise just makes
the brakes hot to charge a battery. Or you use the energy still contained in your hot motor exhaust to run a turbo charger.
At the time of Wilhelm and Friedrich the meaning of the term was the same but in addition it also meant to be generally
energy conscious. You can do that only after the concept of energy is clear.
|
|
 |
Siemens realized that in burning regular fuel for producing heat, a part of the energy produced
is used to heat up the fuel itself. There is not much you can do about that as long as you burn solid matter - wood, charcoal,
normal coal, coke - but if you used the town gas
that resulted from coke making, you could pre-heat it
for free if you used the heat contained in the hot exhaust gases of any furnace treating pig iron.
The Siemens brothers,
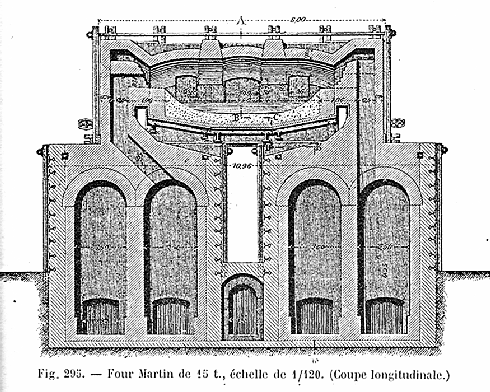
in essence, came up with the idea of a heat exchanger. Run the hot exhaust gases through a chamber filled with stacked bricks,
and you heat up the bricks. Then run your process gases through these hot bricks and you heat up the gases. Have two heat
exchanger units and switch back-and forth from one to the other. Here is the classical drawing showing what the whole contraption
looks like: |
|
|
|
 |
I have looked at that drawing many times and could never make head or tail of
it. If you can, you have my heart-felt congratulations. If you cannot, you might profit from activating the link to the
large schematic picture. There the concept is easy to understand. |
|
 |
Let's just say that a working Siemens - Martin regenerative furnace (always of the reverberatory kind) could get a substantial mass of iron / steel
not only up to the melting point of pure iron at 1538 oC (2629 oF) but up to 1800 oC (3272
oF)! And it did so with rather little fuel. That's an impressive achievement - but is it useful? What is the
advantage for fining pig iron and making steel? Can you see it?
On the surface it is not all that obvious why the Siemens
- Martin process is so much better than the Bessemer and Thomas processes. Looking a bit more closely, however, produces
a long list of advantages:
- You can take the carbon out of pig iron now by various means because the stuff always stays liquid and doesn't solidify with decreasing carbon content. There is no entrapped
slag either because of this.
- Same thing for silicon, phosphorous and so on. In essence you can do standard "chemistry" because everything
is always liquid.
- You can put stuff into the liquid for the same reasons. It will distribute quickly
and uniformly because everything is liquid.
- In particular you can put scrap iron into the liquid. That is an enormous
advantage because it allowed for the first time the recycling of
scrap iron, of which rapidly increasing amounts were generated.
- You can take a spoonful out at any time and analyze it (quickly) in the on-site lab. That told you what kind of mix
you had concocted, and thus also what you had to do to get what you want. Too little carbon? Throw some in. Too much carbon?
Blast some air into the stuff. Too much phosphorous? Add some quicklime. A little manganese on the side? No problem Sir;
coming right up.
No more cooking blind and without tasting!
- You didn't burn (oxidize) as much iron as in the other processes and used less fuel - that means less money is needed.
- Siemens - Martin furnaces could be made bigger and bigger without much problems (unlike the Bessemer / Thomas converters
that needed to be moveable). There was economy of size! Capacities of 50 tons to 100 tons were the rule, but even 500 tons
were possible; just look at this monster. Compare that to the 5 tons
or so of a Bessemer or Thomas converter
A few details (including the problems) can be found here. But
now it is time to progress to the Martin's. |
 |
The Internet offers very little about Pierre-Émile Martin and his father François Marie Emile Martin - except that the Siemens
- Martin furnace translates to "four Martin" in French (of course). I'm not going to help the French by providing
extensive insights into the role of the Martins but will give only hints. |
|
 |
The Siemens brothers actually could not produce liquid
steel since in their prototypes the linings of the furnace melted around 1600 oC (2912 oF). If you
look at their contraption, it's not something easily and cheaply build and re-build. Experiments must have been expensive
and slow. Some experience with iron and steel technology (that the brothers did not have) would also have been quite useful.
Pierre-Émile Martin and his father François Marie Emile, two French iron and steel experts, liked the
concept, took out a licence and began to work. They solved the "melting brick problem" by using better material,
and they probably added a lot of other improvements.
The Martin's succeeded in making very good steel with the regenerative
furnace; their product was awarded a (then very prestigious) gold medal at the 1867
World Exhibition. Siemens got one too for the invention of the furnace! The Martin's also filed patents of their own and
- unavoidably - had them challenged by some Siemens. The ensuing litigation reduced Martin to virtual poverty while others
were making large profits using "his process". Finally, when Martin was 83 years old, the Comité des Forges
de France (“Ironworkers Guild of France”) instituted a fund for him that was supported by all of the principal
steelmaking countries. Barely one week before Martin’s death, the Iron and Steel Institute, London, honored him with
its Bessemer Gold Medal. |
 |
The Siemens - Martin process dominated the World production of iron / steel for
more than 80 years. Of course, it also had some drawbacks (like being slow) and after about 1970 "oxygen-blast steel"
and "electro steel " took over; see above. |
| |
| |
| |
Oxygen-Blast and Electro Steel |
 |
We are now entering the era of modern steel making and I will
keep it short. |
 |
Oxygen-blast steelmaking (also known
as "Linz-Donawitz-Verfahren steelmaking" or "oxygen converter
process") simply blows oxygen and not air on
the liquid pig iron. Linz and Donawitz are Austrian cities, and Austrian companies first employed this process. The main
guy pushing this technique was the Swiss engineer Robert Durrer
(1890 – 1978), who studied and worked as a professor at the Berlin Institute of Technology until 1943. |
|
 |
Well, oxygen should be more effective than air, that's obvious, but how to get
the stuff in quantity and cheap? Carl von Linde (German) made
it possible by extracting liquid oxygen from liquid air by distillation in 1895; around 1930 the process provided plenty
of cheap oxygen. But that's a long story in itself. Suffice it to say that the Linde Group,
founded by Carl von Linde, is presently the world's largest industrial gas company.
After the second world war, the
Austrian Iron and Steel mills in Linz, making the modern kind of the Ferrum
Noricum, were majorly kaputt, owing to allied bombs. The decision was to rebuilt by developing and utilizing a new process:
oxygen blasting. As simple as is sounds, it took some dedication to overcome the problems. The first experiments 1949 (my
birth year) yielded this result: "Der hergestellte Stahl war miserabel" (the steel produced was abysmal). The
rest is history. |
 |
You can use all the methods covered above, e.g. blow oxygen through a Thomas converter
and so on. There are several advantages to using oxygen instead of air, suffice it to mention that you do not get nitrogen
into the steel, typically not a good thing, and that oxygen blasting makes processing considerably faster and cheaper. |
 |
Electro Steel is made in an electric arc furnace. The principle is simple. Put graphite electrodes (usually three) right over
the (solid or liquid) pig iron and run a substantial current from the electrodes to the iron. For doing that you need to
ignite an electric arc between the graphite and the iron, and there it gets rather hot. Temperatures up to 3500 °C
( 6332 °F) can be reached, allowing alloying with very high melting point metals like tungsten (W) or molybdenum (Mo).
In essence, you feed the energy needed to increase the temperature of the iron into the system by electrical means and thus
with very high efficiency. |
|
 |
You can blow in some oxygen to take carbon out, remove other stuff by forming proper slags,
dissolve scrap iron and so on. The process is faster than the Siemens Martin process, and a furnace can be far more compact,
allowing easier access and so on.
However, you better have a sizeable electric power plant nearby. An electric arc furnace
is not something you can plug into your kitchen outlet, it needs serious juice for running. Also, you better know how to
handle powerful electric arcs; not an easy thing to do. It's like trying to control a flash of lightning. It is not very
cheap either.
Electric arc furnaces nowadays are typically used for producing expensive high-quality steels. 29 % of
the steel produced world-wide comes from electric arc furnaces right now - about 450 Mio tons! |
| |
| |
© H. Föll (Iron, Steel and Swords script)