|
9.4.2 Phosphorous Steel - a Chaotic Succession |
 |
Originally I planned to call this module "Phosphorous in Ancient Times".
After quite some time of looking into the issue, I concluded that the new headline is better. So I won't go smoothly from
one point to the next, pointing out how they are all related, that we understand it all, and that my brethren and I are
really superior guys who you should send large amounts of money to or at least worship a bit.
I'll just give you a
taste treat of what is out there. Since the topic "phosphorous in iron" will come up several times again in various
contexts, I will on occasion also give links to one of the modules ahead.
Let's
start with a quote from Samantha Rubinson's 2010 thesis, p.280: |
|
 |
"Phosphoric iron was a commonly used alloy in the Early Medieval
Britain. 66% of the artifacts examined in this study contained significant
amounts of phosphorus in their iron alloys.
This research found that phosphoric iron was one of the three major iron
alloys used in Early Medieval Britain. This result indicates that phosphoric iron was present in much larger quantities
than previous studies (McDonnell 1987b, McDonnell 1992, Tylecote and Gilmour 1986) were able to identify. This discrepancy
was due to the limitations in identification of the alloy. .... These results indicate that many of the preconceptions of
phosphoric iron are invalid."
1)
Consider this, and in addition that phosphoric
steel was nearly always used in the heydays of the pattern-welded sword (300 AD - 800 AD) on the continent. A first major question emerges: |
| | |
|
|
|
Did our forebears make phosphoric iron intentionally?
|
|
| |
| |
 |
Did our forebears like to work with the stuff?
So far everybody (including me) more or less assumed without much discussion that phosphorous in (old) iron got in there
unintentionally because phosphorous-bearing iron ore was used in smelting. Samantha
Rubinson and others are not so sure anymore. Maybe the old ironmongers added phosphorous-bearing stuff like bones (containing apatite or calcium phosphate) intentionally to what they put into their smelters? |
|
 |
This questions leads us straight to smelting technologies, the growing awareness that smelting
iron is far more tricky than imagined, and that our ancestors could smelt iron and steel in far better ways than imagined
not so long ago.
We simply do not know enough about the old "bloomery" techniques used before the advent of
the blast furnace - roughly - around 1400. More to that (far more!) in chapter
10.2 |
 |
There is second (minor) question hiding in that quote: Who discovered when and
where that ancient steel was often phosphoric steel? |
|
 |
Samantha quotes work of McDonnell, Tylecote and Gilmour from about 1985. I feel certain that
there are older sources but don't feel like looking into that right now.
That feeling is partially due to the expectation
that early researchers stumbling on to phosphoric iron could not possibly have gotten everything right. Getting a grasp
on phosphoric iron needs more than seeing some ghosts, interpreted as
phosphorous-rich areas, after defect etching. And that "more" was not available until 20 years ago. |
 |
This leads straight to the next point, raised more recently by Samatha Rubinson
but also by others: |
|
 |
Could it be that "ghost structures" or other etching results that were interpreted
as caused by phosphorous were actually due to other elements, e.g. arsenic? Or maybe a mixture of elements?
The general
answer is: Yes, it could be. After that it becomes muddy. While there are indications that there is such a thing as ancient
arsenic iron or phosphoric-arsenic iron, it is to early for me to go deeper into this. |
 |
Of course, one needs also to consider the negation of the question above: Could
it be that there is phosphorous / arsenic / etc. in the steel and no
ghost structures are produced? |
|
 |
Yes, that could be; says Samantha ("In many cases no indicators
were present to identify the presence of phosphorus in the ferritic microstructure"). Ghosts are tricky (the
British should know) and not always around when they should be. Again, a topic that needs far more research before much
more can be said. |
 |
While a steel containing some phosphorous and
some arsenic can be expected to behave roughly like a steel with only phosphorous at some higher concentration (the sum
of both), the same can not be true for a steel that contains phosphorous and some carbon.
While we do know a few things about P,C- steel, I won't go into that topic except to note that this kind of ternary alloy
was rather common, at least as far as studied by Samantha Rubinson. Here is what she found: |
| |
|
| |
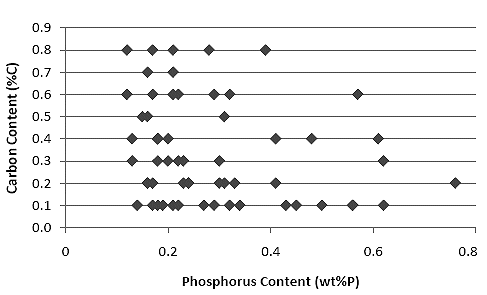 | Phosphorous and carbon concentrations in early medieval
samples from England | |
Source |
|
| |
|
|
 |
There is no particular trend visible; almost everything goes. Note on the side that almost
pure carbon steels come with concentrations between 0.1 % and - hypereutectoid (!) 0.9 %. |
 |
Now let's look at a few more interesting points: |
|
 |
There seems to be some consensus that phosphoric iron is easier to forge and to work with than carbon steel. It seems
to be particular beneficial to wire drawing. That
is not only claimed in the link given above but seems to be a kind of diffuse common knowledge. Of course, we will only
get to know particulars if experienced smiths do specific and well-designed experiments. |
|
 |
Phosphoric iron can be harder then wrought
iron. That is no surprise, we know already that it is one of the three outstanding solution
hardeners. However, this necessitates that the phosphorous is atomically dissolved and not precipitated as the phase
diagram demands for nirvana (equilibrium). In addition, it should not be concentrated in grain boundaries either. Here is
what Samantha found (turned 90o and augmented by me) in comparison to what well-known Hungarian / Czech Republic
researchers 1) found: |
| |
|
| | |
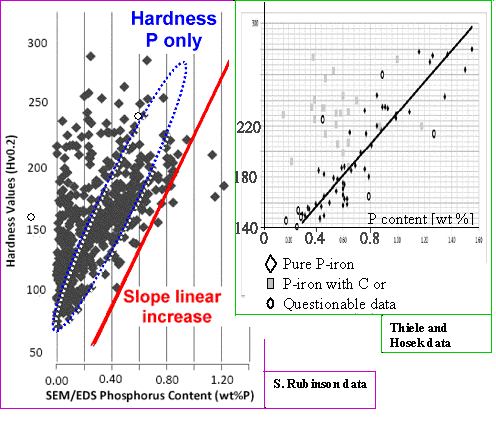 | | Hardness versus phosphorous concentration |
| Source |
|
|
| |
| | |
Samantha Rubinson's data are a bit strange. While quite a few points inside the
range outlined in blue would fall on the expected linear increase line (that means doubling the P-concentration would double
the hardness; the red line shows the slope), many samples are much harder than one would expect from only their phosphorous
content, and few are softer. That could only mean that
- there was no appreciable precipitation of Fe3 P, and
- there must be additional hardening, probably because some carbon was also present and cementite has formed.
The data from Thiele and Hosek (matched in scale to the data of Rubinson) agree in general terms but not in detail,
showing once more that things are complex. |
| |
Whatever, while a hardness around 200 is not breathtaking if you think about
the cutting edge of swords, it is respectable and quite sufficient for many other steel uses. The ancient smiths must have
been puzzled no end that some initially hard steels could be further hardened by quenching but not always. Well, if an initial
200 hardness was due to phosphorous and not to carbon, quenching cannot produce martensite and thus extreme hardness. |
|
 |
So no Fe3P precipitates in
all the samples above?. That could well be the case. The precipitation of phosphorous is difficult because it has to happen
at relatively low temperatures. We might establish a "no Fe3P precipitates" rule at this point. In
fact, Samantha Rubinson, while spelling out "phosphoric iron" a few 100 times in her thesis,
never ever mentions Fe3P precipitates.
But we should be careful about generalizations. Buchwald has encountered
old steel that did contain iron-phosphide precipitates (and thus was rather soft for its phosphorous concentration). Here
is the relevant picture: |
| |
| |
| |
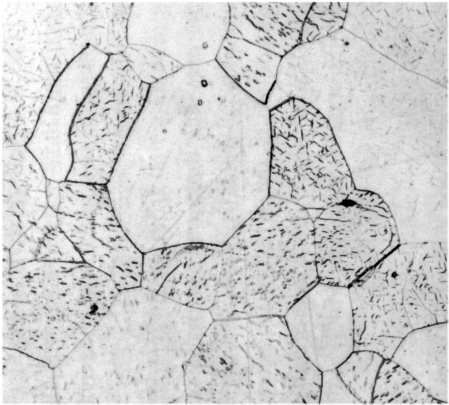 | | Iron-phosphide precipitates in old steel |
| Source |
|
| |
| |
| |
|
The little black dashes are iron-phosphide precipitates according to Buchwald.
Why that exception to the "no Fe3P precipitates" rule? Well, maybe that sample has gone through some
annealing that just happened to be perfect for inducing precipitation. Maybe most of the precipitate growth happened in
the 1000+ years the sample was hanging around at room temperature? Maybe those "little black dashes" aren't Fe3P
precipitates but some stuff containing arsenic? (Buchwald couldn't yet know about that possibility).
Your guess is
as good as mine: We need to wait until more research clears up the topic. |
|
 |
What about arsenic steel? Is there such a
thing as iron with (almost) only arsenic (As) as alloy element? Well - not quite. Samantha Rubinson did find a few pieces
with high arsenic concentrations of up to 1 %, but always mingled with the other stuff. She figures that it was in there
unintentionally - in contrast to phosphorous.
So arsenic is not interesting? Note quite so. We will encounter it again
in the context of the so-called "white weld
lines". This is another still mysterious topic that needs elucidation.
All things considered, we do not need
to be overly worried about arsenic in iron. One must keep an open mind, though. |
|
 |
Why is phosphoric steel more corrosion
resistant? Because it is more homogeneous than carbon steel. There are no cementite particles sticking out into
the surface that can't be covered by a passivating oxide. In addition, some corrosion
inhibiting phosphates might form as outlined before. Note that phosphoric iron
is not "stainless", it just rusts slower than comparable carbon steel. The practical
consequences may have been considerable, however.
No big mystery here - although the details are far from clear.
|
|
 |
As the last point, we need to consider the "whiteness"
of phosphoric iron or "silvery iron" as it was sometimes called according to Samantha Rubinson. All I can tell
you is that I know a lot about why materials appear more or less colored if viewed with normal light, that it can't be explained
in simple terms, and that I do not have a clear idea about how a little bit of phosphorous changes the appearance of a piece
of iron.
Of course, you never look at a piece of iron but always at a piece of iron covered by some thin
layer of oxides. These oxide influence the "color", the appearance to the naked eye. So maybe the oxides
on phosphoric iron are different from those on regular steel an "make" the silvery-white appearance? I have a
clear answer to that: I don't know. |
 |
All things considered, phosphoric iron has quite a lot going for it. It has two
big disadvantages, however: - It promotes brittleness, in particular cold-shortness.
- It does not allow high hardness levels since no martensite can be formed.
The second point is not a problem for many applications but cold-shortness is. Or is it?
To be sure: phosphorous
does cause cold-shortness and the "silvery iron" or whatever else it was called in ancient times was known to
become brittle when cold. At least most of the time. We are allowed to wonder, however, if there might have been ways to
make phosphoric iron from some bloomeries, containing slag particles and a little bit of this and that, less brittle than
the "common" variants if treated "right"?
If we look into cold
shortness scientifically, and into the influence of small amounts
of impurities and alloy atoms, we find that we look into an extremely complex issue. Phosphorous causes extreme cold-shortness
and parts of the mechanism behind that is its tendency to precipitate into grain boundaries. Avoid that and you get non-brittle
or ductile phosphoric iron? |
|
 |
To quote S. Rubinson once more: "In most archaeological
artifacts there is no evidence of brittleness in the phosphoric iron microstructure ... . Vallbona (1997) suggested that
this was the result of the presence of slag".
Maybe the ubiquitous slag particles in bloomery steel are
good in this case? Hard to know because modern steel that is typically used for experiments does not have slag inclusions.
Well, modern phosphoric iron has a champion on its own, E. Balasubramaniam
, who we have met not that long ago. He looked into the issue in some detail: "In
order to understand the possible method by which the ancient blacksmiths overcame the problem of embrittlement in phosphoric
irons, a very careful study of the microstructures of several ancient Indian irons (dating from the 5th Century AD up to
the 19th Century AD) was undertaken."
I give you the whole 2003 paper in this link. |
|
 |
Enough! Throughout the history of iron up to modern times, phosphorous in steel
has provided for much trouble but was nevertheless extensively and deliberately used.
The last word to that topic needs
yet to be spoken! |
| |
| |
© H. Föll (Iron, Steel and Swords script)


